Introduction
Endogenous opio-peptides act as hormones and neurotransmitters.1 Exogenous opioids have wide-ranging, profound effects on neuroendocrine physiology. Despite a label change by FDA in 2016, only 66% endocrinologists and 25% non-endocrinologists were aware of these effects.2–5
20% of patients in primary care have chronic non-cancer pain, many of whom are prescribed opioids.6,7 Table 1 lists some common opioids.8
Methods
This is a narrative and scoping review.
PubMed search for ‘opioid induced endocrinopathies’ yielded 227 results between 2014 - 2024. 61 relevant articles were extracted. As there is a dearth of randomized controlled trials on opioid-induced endocrinopathies, observational studies, review articles and relevant basic science articles were included. Articles including critically ill patients were excluded. References from these articles were also searched.
A second PubMed search in January 2025 for ‘distribution of opioid receptors in the human body’ returned 29 results, 20 of which were directly relevant.. Abstracts of these 20 articles were reviewed to select the most cogent ones. 7 were read completely.
Google Scholar search for ‘opioid-induced endocrinopathies’ from 2018 - 2025 yielded 75 results. After excluding articles not in English, duplicate articles and those not relevantly addressing the search term, 19 articles were short-listed and included.
Selected articles were read completely by the author. Lists are available in Supplementary Appendices 1-3.
Data analysis: Research of studies was iterative and recursive. Relevant data on presentation, mechanism and management of opioid-induced endocrinopathies was extracted by content analysis. A narrative synthesis of the data was performed.
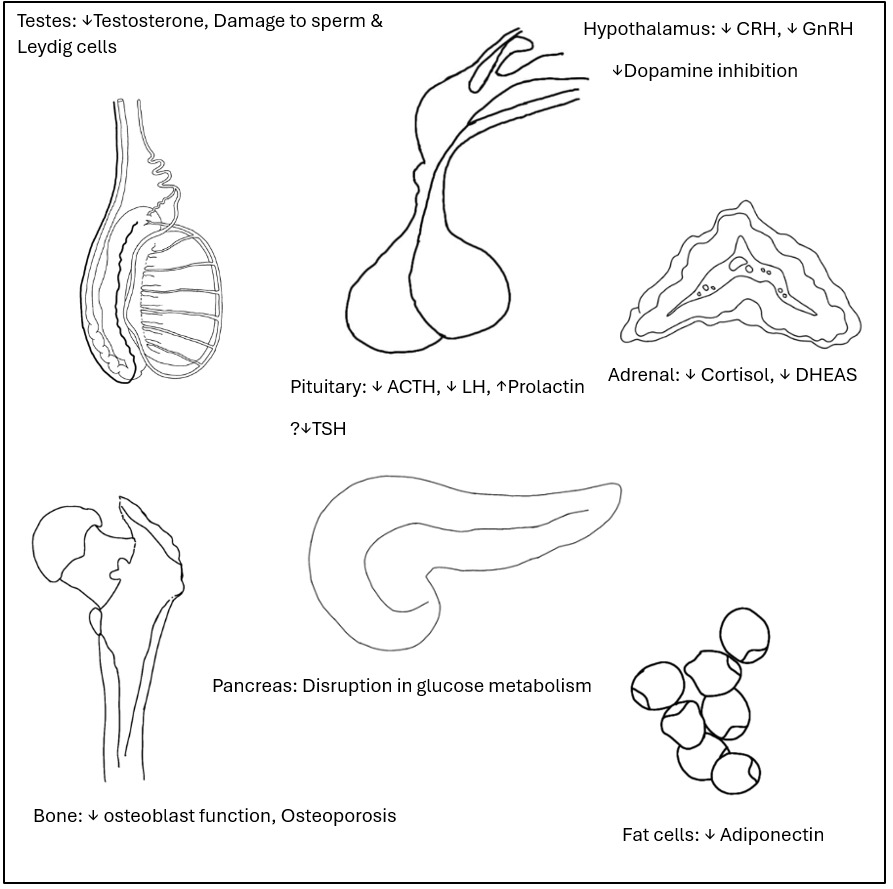
Results (Figure 1)
Opioid-induced Hypogonadism (OIH)
Overall prevalence was 76% in men and 64% in women; prevalence in cancer patients was 21 - 86%5,10
HPG axis suppression occurred with acute and chronic administration of short- and long-acting opioids regardless of route of administration, structure or lipophilicity.11–16
OIH was centrally- and peripherally-mediated. Pulsatile gonadotrophin-releasing hormone (GnRH) secretion was inhibited via µ, δ and ĸ receptors and more pronounced in men younger than 60y.5 Peripheral testosterone metabolism was enhanced.17 Direct deleterious effects on sperm and Leydig cell apoptosis have been reported in animal studies.18 Up to one-third of men on fentanyl patch had low testosterone with high follicle stimulating hormone (FSH), luteinizing hormone (LH) and high prolactin. Morphine suppressed dehydroepiandrosterone (DHEAS).4,19 Partial agonists buprenorphine or tramadol caused less profound HPG suppression.20
A 66% decrease in opioid dose led to rapid restoration of testosterone levels within 30 days.4
In females, menstrual disturbances were frequent (23 - 81%) with opioid use of 30 days.6 Risk of menopause increased. Opioids also induced infertility.21,22
Hypothalamic-Pituitary-Adrenal (HPA) Axis
Prevalence was 4 - 29%.23 Tonic CRH suppression via µ-receptors occurred with short- and long-term opioid use.18 ĸ and δ receptors impacted ACTH release. Oral morphine >50 mg for just 3 days reduced cortisol levels to less than half of baseline.24 Diurnal cortisol rhythm was disrupted.25 Cortisol suppression occurred with long and short-acting opioids, irrespective of route of administration.22,26 Morning cortisol was often low.27 The opioid derivative loperamide suppressed basal and CRH-stimulated ACTH secretion causing clinical OIAI.9 HPA axis suppression occurred with doses of <3 - >20 morphine milligram equivalent (MME).24,25 Adrenal crisis occurred rarely.24
Bone
Morphine suppressed osteoblasts in-vitro.28,29 Intrathecal morphine led to low bone density in men 18 - 45 y of age.23
Disruption of glucose metabolism8
Opioids caused hyperglycemia in non-diabetic subjects in a dose-dependent manner.30 In diabetic subjects, hypoglycaemia occurred.1 Severe, recurrent fasting hyperinsulinaemic hypoglycaemia was reported with methadone; the effect was reversed by dose-reduction or switch to fentanyl or buprenorphine.31
Serotonin Syndrome
Opioids inhibit serotonin reuptake and caused serotonin syndrome when administered independently or concomitantly with serotonin-reuptake or monoamine oxidase-reuptake inhibitors.32
Morphine and codeine stimulated histamine release from mast cells causing pruritus.8
Adiponectin
Opioids decreased adiponectin levels.8 Adiponectin is the most abundant peptide produced by fat tissue and prevents impairment in lipid and glucose metabolism and coronary function.33
Thyroid and Growth hormone (GH) axes23
Opioids decreased IGF-1 in 42.11% on dynamic testing; however this was statistically insignificant.
Basal levels of TSH and free T4 remained normal in all patients.
Prolactin
Acute and chronic opioid administration via any route caused loss of tonic dopamine inhibition.3 Effect was strongest with morphine and methadone.15
Anti-diuretic Hormone / Arginine vasopressin (ADH/AVP)
Opioids suppressed hypothalamic ADH secretion in surgical or laboratory settings without clinical diabetes insipidus.34,35
Non-AVP mediated hyponatremia occurred, especially with tramadol.14
Oxytocin
Morphine and fentanyl suppressed oxytocin levels during the first stage of labour and breastfeeding.14
Discussion
Opioid receptors (Table 2) belong to the G-protein coupled receptor (GPCR) superfamily.36–38 They are ubiquitously distributed. ORL1 is not naloxone-sensitive.1
While cumulative opioid exposure correlates with severity of endocrine side-effects, these can occur with any agent regardless of daily dose. All pharmacologic opioids are µ-receptor-selective rather than µ-receptor-specific. Second, opioid receptors exist alone, in versatile oligomeric conformations with one another and perhaps even with other GPCRs. An opioid produces different effects based on the receptor or homo- or hetero- oligomer it attaches to. Third, the µ-receptor gene exists in many different isoforms due to alternative pre-mRNA splicing.39 Fourth, there is a ‘ligand-bias’: every opioid results in different ‘active’ conformational forms of a receptor with unique downstream intracellular effects.40
It is now known that ‘weak’ opioids can have very potent metabolites. Metabolites vary depending on route of administration. Inherited polymorphisms mean that different individuals will metabolize opioids very differently.14 Thus so-called ‘equivalent’ doses of various opioids cannot be simplistically compared.36
Management of Opioid-induced Endocrinopathies
For non-malignant pain, non-pharmacologic and non-opioid drug treatments should be considered first.41 Opioid-induced endocrinopathies increase morbidity and mortality. Long-term opioids deliver only a small additional benefit in chronic pain.42,43 Patients should be counselled about endocrine side effects before commencing opioids and dose minimized.
OIH and OIAI
Clinical features of hypogonadism (Table 3) and hypocortisolism (Table 4) can be non-specific and hard to distinguish from other causes, both in patients with cancer and those with chronic non-cancer pain. Patients should be screened via history at baseline. The frequency of clinical review during follow-up is undefined but a high index of suspicion should be maintained. Where indicated, biochemical monitoring should be considered. Male hypogonadism can paradoxically increase opioid dose requirement.43
In some cases, judicious hormone replacement can be used. Topical testosterone replacement in men can improve pain control, anemia, libido, body composition, fragility fractures, major adverse cardiovascular events, quality of life and all-cause mortality; however erectile dysfunction, self-reported pain and functional status may not improve.18,27,44,47,48
Testosterone replacement is non-conducive to reproductive function. Clomiphene citrate has been proposed for fertility preservation but remains to be rigorously studied.49
Monthly or bimonthly testosterone testing for several months after dose reduction or discontinuation is suggested.3
OIAI is a diagnosis of exclusion in patients with suggestive symptomatology.50 Patients on >20 MME/day should be proactively monitored for OIAI via history.45 Table 5 outlines diagnostic approach to OIAI.
Interpretation of cortisol results in the setting of opioid use is tricky. Pain, opioids and hospitalization all interfere with diurnal rhythm. Pain stimulates cortisol release. It is unclear whether diagnostic cortisol thresholds in patients without chronic pain are equally valid in patients with OIAI. Pre-test probability and clinical judgement determine who and when to investigate.23 In case of subnormal cortisol levels, stress dose steroid cover should be considered and steroid alerts provided.14,24
As OIAI is often central, aldosterone is not affected and co-syntropin results may be false-negative despite low morning cortisol.46,47,51 Knowledge of locally used cortisol assays and their cut-offs is crucial.52,53 Periodic assessment of HPA axis is prudent after opioid reduction or discontinuation until normal cortisol levels return.48
Prolactin
Prolactin levels should only be checked in the appropriate clinical circumstances. Management approach to hyperprolactinemia or galactorrhea should follow standard guidelines. If no other cause is identified, opioid dose reduction should be attempted. Dopamine agonist treatment or gonadal hormone replacement may be considered if dose reduction is not possible.3
Osteoporosis
Chronic opioid use should be factored into consideration of osteoporosis screening. Management of osteoporosis should follow standard guidelines.54 Risk of falls adds to fracture risk, especially in first month of opioid initiation55
Thyroid Axis and Electrolytes
Thyroid monitoring is not indicated. Serum sodium should be monitored in the first 30 days, especially with tramadol.
The limitation of this review is lack of data from randomized controlled trials and rigorous meta-analyses. There is a need for well-designed studies in this area.
Its strength is review of impact on neuroendocrine and metabolic axes and insight into opioid pharmacogenomics and pharmacokinetics.
Conclusion
Opioid-induced endocrinopathies are associated with increased morbidity and mortality. Patients should be counselled before therapy initiation, monitored during follow up and consideration given to tapering off or reducing opioids wherever possible.