Introduction
Non-invasive ventilation (NIV), including continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPap), and high-flow nasal cannula (HFNC) are important modalities of oxygenation support in acute hypoxemic respiratory failure (AHRF) that avoids the risks associated with mechanical ventilation.1 These methods of non-invasive respiratory support are well-tolerated with mostly mild side-effects, including discomfort, abdominal distension, and claustrophobia.2 Potentially more serious complications have been reported, including but not limited to pneumoperitoneum, pneumomediastinum (PMN), spontaneous pneumothorax, and subcutaneous emphysema (SE) defined, respectively, as pathologic air in the peritoneal cavity, space between the lungs above the diaphragm, space between the lung pleura, and within the soft tissues under the skin.2,3
The mechanism behind the development of pneumomediastinum is described through the Macklin effect, which theorizes that an increase of intra-alveolar pressure, as seen with positive pressure ventilation, can cause rupture and subsequent dissection of air along the lung interstitium into the lower pressure environment of the mediastinum. This air can extravasate into the subcutaneous tissues.4 Air can further travel into the peritoneum through pleuroperitoneal anatomic defects, resulting in pneumoperitoneum.5 Although less common than in mechanically ventilated patients, patients who receive non-invasive respiratory support remain at risk of complications from the Macklin effect as well.2,6 Patients whose spontaneous breathing remains intact can develop dysregulated and intense inspiratory effort and hyperinflation, leading to barotrauma and alveolar inflammation similar to that seen in ventilator-associated lung injury.1,7
PMN and SE are becoming a more recognized complication of non-invasive respiratory support with the onset of the novel SARS-CoV-19 (COVID-19) global pandemic due to frequent occurrence of AHRF in infected patients along with the extensive pulmonary inflammatory changes .3,8–12The much rarer phenomenon of spontaneous pneumoperitoneum has been documented in only several cases.2,12–17 We present a case of a pseudo-pneumoperitoneum associated with non-invasive respiratory support, but uniquely, as part of a triad of pneumomediastinum with extensive subcutaneous emphysema extending circumferentially around the abdomen and down into the scrotum. To our knowledge, this has not been described elsewhere.
Ethical considerations: The patient gave written informed consent for the publication of this case report and associated images.
Case Presentation
A 75-year-old male with chronic obstructive pulmonary disease (COPD) and congestive heart failure (CHF) presented to our emergency department with a chief complaint of acute worsening shortness of breath. As part of the dyspnea workup, computed tomography was obtained of the chest, which demonstrated diffuse interstitial lung thickening from edema with scattered emphysematous cysts [Figure 1]. He was subsequently admitted to our hospital for acute hypoxic respiratory failure and initiated on diuretics and antibiotics for suspected COPD and CHF exacerbation. Continuous positive airway pressure was started with albuterol/ipratropium nebulization every four hours.
The following day, the patient’s respiratory status worsened, and he was started on a high-flow nasal cannula with pulmonology recommending CPAP at night. Lung function continued to deteriorate and on the morning of his third day of admission, the patient was transferred to the progressive care unit. He was on CPAP for a total of four hours that morning with continuous pressure of 15 cmH2O, a high-pressure limit of 35 cmH2O, and a low-pressure limit of 5 cmH2O. During the day, he was on HFNC of 60 liters per minute at 100% fraction of inspired oxygen.
It was noticed that afternoon he had developed abdominal distension and scrotal swelling, for which an immediate screening chest x-ray was performed, showing pneumoperitoneum. This resulted in surgical consultation due to concern for bowel perforation and a stat CT of the abdomen and pelvis with contrast to be obtained [Figure 2]. The CT imaging demonstrated that the appearance of pneumoperitoneum was related to pneumomediastinum and subcutaneous emphysema that extended circumferentially around the abdomen and anteriorly down to the scrotum in the setting of CPAP.
General surgery determined the pneumoperitoneum was not a result of perforation, but rather, due to the use of CPAP. The patient had no peritoneal signs and the pneumatosis and subcutaneous emphysema was determined to be benign. CPAP was considered essential due to worsening of patchy infiltrates on chest x ray thought to be secondary to cardiogenic pulmonary edema due to CHF exacerbation. It was determined the best course of action was to maintain, but limit, CPAP using the same settings two hours a night with HFNC in between. Despite limiting CPAP, the patient’s abdominal x-ray demonstrated worsening of the pneumoperitoneum the following day [Figure 3]. CPAP was discontinued and the patient was maintained on HFNC of 60L on 100% FiO2. He was subsequently discharged to hospice, where the patient subsequently died.
Discussion
Risk factors for the development of PMN/SE in the setting of non-invasive respiratory support have mostly been studied in the setting of COVID-19. Diabetes mellitus, hypertension, and lung disease are the most common comorbid conditions, although the majority of patients have several complicating medical problems, suggesting sicker patients are more likely to develop these complications in the setting of respiratory failure.3,10 In non-COVID-19 patients, distinct risk factors are unclear and have not been elucidated.
The patient in this case has several physiologic risk factors that likely contributed to the development of the reported complications The first includes inflammation in the setting of COPD exacerbation and pneumonia. Second, CT imaging demonstrated interstitial thickening due to edema from CHF, likely resulting in decreased lung compliance and high alveolar pressure.9 Taken together, lung fragility from increased inflammation accelerated impending rupture due to overdistension in the setting of limited compliance, ultimately contributing to the Macklin Effect and the patient’s pneumoperitoneum and PMN/SE.
The patient’s complications occurred in the setting of HFNC and NIV, raising the question of which was the culprit. BiPap and CPAP were used for respiratory support in all instances of pneumoperitoneum reviewed for this case.2,12–17 HFNC use has been associated with PMN/SE; however, it is theorized HFNC is a better modality of respiratory support in these patients as it exerts minimal positive end-expiratory pressure (PEEP), decreasing alveolar overdistension and self-inflicted lung injury.7,9 The positive correlation of higher PEEP with PMN/SE has been observed clinically with patients receiving more aggressive pressure support demonstrating increased complications from NIV.11 Conversely, using HFNC in cases of PMN/SE has been shown to lead to the resolution of these complications. In cases of cardiogenic pulmonary edema secondary to CHF exacerbation, as seen in this case, management has classically involved the application of NIV. The rational relates to one of the primary mechanisms of NIV, which is increased pulmonary pressure reduces preload due to decreased venous return, resulting in decreased pulmonary congestion and edema in heart failure with reduced ejection fraction.18 This rationale is what led the patient’s care team to continue NIV despite suspecting it was causative of the patient’s pneumoperitoneum. However, HFNC has been shown to be an appropriate modality for respiratory support in cases of cardiogenic pulmonary edema as well.19 In the majority of cases reviewed, management of the complications was conservative and often associated with spontaneous resolution, particularly when HFNC was utilized.5,9,13,15,17
Conclusion
Cases of pseudo-pneumoperitoneum, SE, and PMN in the setting of non-invasive respiratory support are rare and may mascaraed as more serious pathology, intimidating the clinician unaware of these complications. Given the critical importance of oxygenation, as well as the benign nature of these complications, respiratory support must be maintained through whichever modality best sustains the patient’s oxygenation when pseudo-pneumoperitoneum is suspected in the appropriate clinical context.

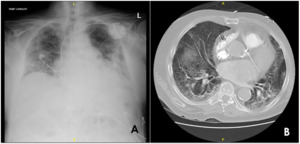
_and_contrasted_ct_scan_of_the_abdomen_demonstrating_(b)_near-circumf.png)


_and_contrasted_ct_scan_of_the_abdomen_demonstrating_(b)_near-circumf.png)
