Introduction
Parkinson’s disease (PD) is a neurodegenerative disease that affects dopaminergic neurons in the substantia nigra, located in the basal ganglia of the brain. This region of the brain is involved in coordinating movement by sending signals down the spinal cord to control muscle contraction, meaning that damage to this region can compromise signaling, leading to the physical symptoms of PD.1 PD is the second most common neurodegenerative disease after Alzheimer’s disease (AD), with a prevalence of approximately 0.5–1% among those 65–69 years of age, rising to 1–3% among persons 80 years of age and older.2 The neuroinflammation, neurodegeneration, and loss of motor function in PD patients can be attributed to the aggregation of alpha synuclein and formation of Lewy body inclusions.3 Alpha synuclein (abbreviated α-synuclein) is a protein normally present in the brain due to its important role in neurotransmitter release.3 α-synuclein has been found to be misfolded and overexpressed in people with PD, which leads to fibril formation and aggregation; in addition to this, α-synuclein’s natural lipid binding properties help explain why it is able to form Lewy Body inclusions (insoluble deposits that form within the substantia nigra of people with PD), which contain several cellular components that have a high lipid content.4 Lewy bodies directly interfere with basic cellular processes, such as mitochondrial functions within neurons and synaptic function/transmission, which leads to poor neurotransmission, resulting in neurodegeneration.4
Dopaminergic neurons release a neurotransmitter called dopamine, which is necessary for gross and fine motor control; in PD patients, these neurons are damaged and unable to produce dopamine, so an artificial supply of it must be provided via dopamine precursors that can cross the blood-brain barrier.5 However, drugs that are used to artificially supply dopamine, such as L-DOPA, have harsh side effects including behavioral/mood changes, involuntary movement such as twitching, and vascular symptoms.6 In addition, these medications fail to slow, stop, or reverse neurodegeneration, deterioration, and progression of PD; thus, researchers have begun shifting their focus to understanding the pathways that lead to aggregation of α-synuclein and formation of Lewy bodies as a potential target for therapies that could slow, stop, or reverse neurodegeneration in PD patients.7 By discussing the chemical structure of α-synuclein, how unique properties of α-synuclein contribute to abnormal aggregation and formation of Lewy bodies in PD patients, and reviewing novel therapies that have been developed to address Lewy body neurodegeneration, this review aims to identify and evaluate the effectiveness of possible PD treatments that focus on slowing, stopping, and/or reversing neurodegeneration and deterioration.
Normal vs Pathogenic α-synuclein
α-synuclein, the protein in the synuclein protein family associated with PD, is an intrinsically disordered protein (IDP), or a protein that lacks a fixed 3D structure, which shows that it can play different roles based on cellular locations and can be influenced by different factors.8 α-synuclein is concentrated in presynaptic terminals and has an affinity to bind to lipids, phospholipids (especially those in plasma membranes, mitochondrial membranes, and axonal transport vesicles), and several proteins.9
α-synuclein has 3 distinct regions: the N-terminal region, the NAC region, and the C-terminal region.3 The N-terminal region of α-synuclein is an amphipathic region (contains both hydrophilic and hydrophobic parts) made up of several unique amino acid repeats. This region has a tendency to adopt α-helical secondary structure, which also reduces the tendency of α-synuclein to form β-sheets; the formation of β-sheets drives the process of aggregation and neurodegeneration.10 This is important to understand because although α-synuclein’s interaction and binding with lipid membranes is crucial to its function as discussed above, it can also lead to structural changes that cause aggregation and amyloidogenesis (formation of insoluble amyloid deposits); to prevent this in healthy brains, the α-helical structures in the N-terminal region have been found to stabilize the protein and prevent aggregation by stopping the formation of pathogenic β-sheets.11
In pathogenic α-synuclein, scientists have discovered that the mutation A30P decreases the tendency of the N-terminal region to form α-helices, and the A53T mutation causes this region to have a preference for extended, β-sheet conformations which induces α-synuclein aggregation and onset of neurodegeneration.11,12 In addition, environmental condition changes can cause local acidosis at sites of inflammation and stress, leading to the α-helical structure of the N-terminal region to elongate into pathogenic β-strands.13
The core region of α-synuclein is known as NAC; out of the three synuclein proteins, the NAC region is only found in α-synuclein and it is hydrophobic.8 This region gives α-synuclein the overall ability to generate β-sheets and is highly amyloidogenic (causes α-synuclein to have a higher tendency to form amyloid deposits).14 Because the formation of β-sheets drives the process of aggregation and neurodegeneration, the NAC is surrounded by the N and C-terminal regions to minimize its exposure to extracellular solvents and other molecules, preventing aggregation in healthy brains.15
The C-terminal region is the final region of α-synuclein, made up of an acidic tail that is highly negatively charged. The interaction between the C-terminal domain and the NAC region of α-synuclein inhibits aggregation through electrostatic repulsion, and the N-terminal and C-terminal regions surround and protect the NAC region.8 Truncations of the C-terminal region caused by environmental stressors have been found abundantly in PD patients, and these truncations induce aggregation because the region is not long enough to perform its protective functions and prevent the NAC region from interacting with other molecules and forming β-sheets.16 Both the N and C-terminal regions are potential targets for novel immunotherapy-based PD treatments because of their unique, easily identifiable chemical structures; in order for these treatments to be effective, a deep level of understanding of the chemical structure and pathogenic modifications within the N and C-terminal regions is needed so that highly specific antibodies and immunotherapeutic drugs can be designed.17
α-synuclein Aggregation and Lewy Body Formation
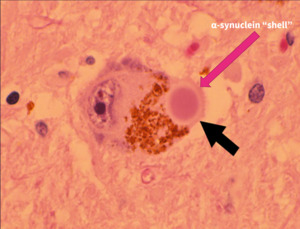
The pathological hallmark of PD is the presence of Lewy bodies (LB) within dopaminergic neurons, which are the main drivers of neurotoxicity and neurodegeneration in patients with the disease.18 Although misfolded and aggregated α-synuclein is a principal constituent of Lewy bodies, studies have found that these deposits contain many other cellular components, including lipid membranes and organelle fragments.4 The core of LBs is composed of lipids and dystrophic mitochondria, surrounded by an aggregated α-synuclein shell.19 Dissecting the molecular events associated with LB formation and how they contribute to neurodegeneration provides a powerful platform for evaluating therapeutics that target α-synuclein aggregation and LB formation for the treatment of Parkinson’s disease.
α-synuclein aggregation has been proven to be the starting event for LB formation.20 The mutations in the N-terminal region and acidosis due to environmental and oxidative stress within both the N and C-terminal regions induce pathogenic misfolding and formation of β-sheets.20 Mutations and chemical changes due to stress in the N-terminal region result in a “broken helix” conformation, meaning that this region will no longer form protective α-helices and instead form β-hairpin structures that form amyloidogenic β-sheets.21 Oxidative stress causes truncation of the C-terminal region, which was found to cause increased formation of strongly twisted β-sheets compared to normal, wild-type α-synuclein.22 When the N and C-terminal regions are mutated, they are no longer able to perform their protective functions (Figure 2), which allows the amyloidogenic NAC region of α-synuclein molecules to interact and aggregate. Understanding of α-synuclein aggregation prior to LB formation is crucial because pathogenic α-synuclein can rapidly spread to other cells once aggregation starts, advancing neurodegeneration; this process is a target for novel PD treatments as an attempt to slow and/or stop LB neurodegeneration.23
LBs are highly complex and heterogeneous assemblies, and since it has been shown that α-synuclein can aggregate into fibrils, scientists hypothesized that α-synuclein may be capable of irreversibly capturing other cellular components through liquid‒liquid phase separation (when a cellular solution separates into dense, liquid droplets and a dilute solution that those droplets are in) before forming a LB inclusion.25 In healthy conditions, liquid‒liquid phase separation is reversible and used for many cellular functions, however, upon dysregulation, liquid droplets can mature into gel-like deposits; in PD, this dysregulation causes the formation of insoluble Lewy bodies.26
Multiple interconnected pathways work alongside α-synuclein misfolding and aggregation, leading to LB formation. To begin, failure of autophagy (removal of unnecessary or dysfunctional components through lysosomal mechanisms) within neurons causes accumulation of dysfunctional, toxic substances.28 Several genes contribute to autophagy failure. Mutations in genes coding the ubiquitin-proteasome system (UPS) lead to accumulation of misfolded α-synuclein (Figure 4, D) because UPS removes unwanted proteins inside the cell.23 Mutations in genes encoding lipid-degrading enzymes and genes causing mitochondrial dysfunction lead to an increased abundance of lipid droplets, which are known to overwhelm autophagy (Figure 4, A and B); ultimately, autophagy failure (Figure 4, C) causes a cycle where the lipid droplets, dysfunctional mitochondria, and misfolded α-synuclein accumulate and are irreversibly captured through liquid-liquid phase separation.29 As discussed earlier, this process eventually leads to the formation of insoluble gel-like deposits, which are also known as LBs.
Novel Treatments for Parkinson’s Disease
Current treatments for Parkinson’s disease provide symptomatic relief by replacing dopamine in affected brains, however, they do not slow or stop the progression of PD and do not promote growth of new dopaminergic neurons in the substantia nigra.5 Thus far, studies have focused on 2 major avenues for new PD treatments: treatments that target α-synuclein aggregates/Lewy body assemblies and treatments that focus on replacement of lost dopaminergic neurons in the brain.
Targeting α-synuclein aggregation and Lewy Body Inclusions
The use of antibodies (immunotherapy) to target and clear α-synuclein aggregates or the upregulation of genes that encode autophagic mechanisms to destroy dysfunctional proteins, lipids, and mitochondria are the two main PD therapies that specifically target α-synuclein aggregation and LB inclusions.7
Immunotherapy against α-synuclein is a promising novel treatment strategy for PD because of its versatility; antibodies can be used to target small and large aggregates before they become a part of LBs. Immunotherapies have been used to target extracellular α-synuclein and were shown to reduce α-synuclein aggregation and behavioral deficits in mice.28 More specifically, antibodies were directed against epitopes (part of an antigen molecule that an antibody attaches to) near the N-terminus and C-terminus of aggregated forms of α-synuclein; because the chemical structures of the N and C-terminal regions are known and pathogenic modifications for each region have been well documented, these antibodies have been designed to have a high specificity for α-synuclein and will not accidentally target other proteins.30 Results from clinical trials have shown a 96.5% reduction in the concentration of extracellular α-synuclein aggregates, and doses were well tolerated with no serious side effects.31 As discussed earlier, misfolded α-synuclein can rapidly spread to other neurons, but because the antibodies are able to effectively clear misfolded α-synuclein, the spread of aggregates to other neurons and neurodegeneration will be significantly reduced.
Although antibodies are invaluable tools for treating PD, their high molecular weight impairs their passage through the blood-brain barrier (BBB).30 Therefore, gene-engineered antibodies called intrabodies and nanobodies have been created; by only expressing coding regions for antibodies, they are significantly smaller, and the full length antibody can be created in the brain itself.7 Additionally, they can be synthesized in large quantities by bacterial or yeast systems, making them more efficient and economical.30
As discussed earlier, several cellular processes, particularly autophagy failure, contribute to the accumulation of dysfunctional cellular components that form insoluble Lewy bodies, leading to neurodegeneration. Thus, novel treatments that target genes encoding autophagic mechanisms are of interest. Enhancement of activity within these systems will reduce mutated α-synuclein levels, lipid droplet accumulations, and levels of dysfunctional mitochondria, helping prevent LB formation.7 A number of drugs have been investigated to activate autophagy.30 For example, rapamycin, a drug that typically works as an immunosuppressive agent, can inhibit mTOR to induce autophagy; mTOR is a protein kinase that contributes to the downregulation of apoptosis and autophagy.7 By inhibiting mTOR, apoptosis and autophagy will no longer be downregulated, helping clear dysfunctional cells and cellular components. Thus, rapamycin and rapamycin-related drugs have neuroprotective effects because they can promote clearance of misfolded α-synuclein and other toxic cellular components through the induction of autophagic processes.32 However, rapamycin lacks specificity and acts on other essential pathways involved in immunosuppression, and more research/trials needs to be conducted in order to determine and test other compounds that can effectively induce autophagy in specific, targeted areas of the brain.30
Astrocyte Reprogramming to Replace Dopaminergic Neurons
In order to rebuild the neurons that were lost to PD neurodegeneration, a renewable source of dopamine-producing cells that can integrate into the host brain and survive for years is required.7 These criteria are met by stem cell-derived neurons; more specifically, the reprogramming of astrocytes can be used to develop functional dopaminergic neurons.5 Astrocytes are specialized glial cells (non-neuronal cells in the central nervous system (CNS) that do not produce electrical impulses) that have many complex functions to maintain the CNS (Sofroniew et al., 2010). The developed CNS has a certain number of neurons that can be permanently lost or damaged from neurodegenerative diseases, and the brain regions relevant to PD have very limited neurogenesis (creation of new neurons) in the adult period, so treatments cannot rely on the growth of new dopaminergic neurons.5 Because they are abundant and can easily switch cell fate, astrocytes can be reprogrammed into functional dopaminergic neurons and replace the ones that have been degenerated.33
To reprogram the astrocytes and fibroblasts, the RNA binding protein PTB was suppressed; PTB suppresses numerous neuronal genes required for neuronal maturation, thus, downregulation of PTB generates functional, mature neurons. During conversion, typical astrocytic genes were successfully suppressed while neuronal genes were induced, giving rise to neurons expressing dopaminergic neuron specific genes.33 When in vivo astrocyte reprogramming was performed in a mouse model of PD, researchers found new, successful dopaminergic neurons in the substantia nigra and increased dopamine levels.5
The main concern of the use of stem cell-based therapies for PD is the potential for tumor formation, which may occur due to overgrowth of the graft or the presence of mutations in genes in the grafted cells; however, tumors have not been observed with improved protocols.5 Secondly, there is a risk for negative immune responses once a graft has been introduced to the brain.5 Specifically, the innate immune system has been shown to react to the vectors (DNA molecules that artificially carry foreign genetic material into another cell that can be replicated and expressed within that cell) that are used to reprogram astrocytes. To solve this issue, several approaches could be adopted; for example, medication-induced immunosuppression could reduce the immune responses against the vector antigens. Thus, if stem cell-based treatments are to be successful, investigation of safety and thorough patient monitoring after the initial graft is crucial to ensure that the newly programmed neurons are surviving, repopulating in the patient’s brain, and not presenting a risk of immune reaction or tumor formation.
Conclusion
Over recent years, the advancement of knowledge about the pathology of PD has led to monumental developments and discoveries that give the potential to be able to slow, stop, or reverse such a debilitating disease and improve patient’s quality of life. Specifically, details about the chemical structure of normal and pathogenic α-synuclein and the pathways that lead to LB formation have allowed for researchers to develop specific targets for novel PD therapies. By targeting α-synuclein aggregation, LB formation, and degenerated dopaminergic neurons, novel PD treatments have the potential of not only alleviating symptoms but also halting progression of the disease and allowing patients to regain function. Both avenues for PD treatment (α-synuclein/LB aggregation and astrocyte reprogramming) have immense strengths. Treatments that focus on α-synuclein aggregation and LB formation are highly specific, making them viable and effective therapeutic options. In addition, they employ many established techniques for immunotherapy and gene regulation, including vaccination, antibody infusions, and existing drugs used to treat various other neurological conditions. Replacement of degenerated dopaminergic neurons provides the opportunity for PD patients to regain functions that they lost due to neurodegeneration. Because neurons are not actively regenerating and dividing, neurodegeneration permanently damages and causes loss of function in the brain; thus, being able to reprogram astrocytes to be functional neurons that can replace the ones that were lost may allow patients with PD to make partial or complete recovery from this disease. It must also be noted that these treatments can effectively work together as a combined therapy; replacing lost dopaminergic neurons via astrocyte reprogramming will allow for patients to regain normal brain function, and immunotherapies and gene regulation therapies will help clear and prevent α-synuclein aggregation and LB re-formation, which will help preserve the new neurons that have been integrated into a patient’s brain. Although both avenues for treatment require further clinical trials and testing in human subjects, they both hold significant promise in the future of PD treatment and neurology.


_the_nac_region_is_protected_by_the_n_and_c-terminal_regions__this_helps_preven.png)



_the_nac_region_is_protected_by_the_n_and_c-terminal_regions__this_helps_preven.png)


