Introduction
Background
Supplemental oxygen therapy is a cornerstone in the management of critically ill patients. However, the optimal oxygenation target in mechanically ventilated patients remains controversial. Selecting either a conservative or liberal oxygenation strategy can increase the risk of hypoxemia or hyperoxia and their secondary consequences.
The effects of hypoxia and hyperoxia are well documented. For example, severe hyperoxia was found to trigger a marked inflammatory response and oxidative lung injury in rodents receiving mechanical ventilation.1 Similarly, another trial discovered that just four hours of mechanical ventilation with induced hyperoxia was sufficient to trigger the development of oxidative lung injury in rats.2
In contrast, hypoxemia is associated with numerous adverse outcomes as well. The primary concern in hypoxemia is decreased oxygen-carrying capacity of erythrocytes, and secondary end-organ ischemia. Clinical and epidemiologic studies have demonstrated that severe and sustained hypoxemia is associated with increased ICU mortality3 as well as ischemic complications of vital organs such as the heart and brain.4
Rationale
Despite longstanding concerns regarding the potential adverse effects of hyperoxia (worsening of oxidative injury) and hypoxia (end-organ damage) in mechanically ventilated patients, there have been limited studies comparing the outcomes in liberal versus conservative arterial oxygen targets. The few randomized controlled trials (RCTs) that do exist, were relatively underpowered and focused almost exclusively on mortality. In addition, these smaller studies lacked the statistical power to detect differences in more patient-focused outcomes such as ventilator-free days (VFD-28) or length of ICU stay (ICU-LOS). Therefore, there is an urgent need to identify an oxygenation strategy that balances safety and efficacy, optimizing outcomes beyond mere survival.
Since 2019, four large-scale trials (ICU-ROX, PILOT, ICONIC and UK-ROX) have rigorously explored these two oxygenation strategies. However, to date, no synthesis has been conducted that examines patient centered outcomes such as VFD-28, mortality and ICU LOS on a large scale. These outcomes were selected due to recent evidence that highlights the adverse effects associated with prolonged mechanical ventilation such as ICU-acquired weakness,5 diaphragmatic muscle atrophy,6 ventilator associated pneumonia,7 long-term decline in quality of life8 and delirium.9 Therefore, it is highly relevant to determine if patients can be liberated from mechanical ventilation at an earlier time using a different arterial oxygen target to avoid adverse outcomes.
Current large-scale quality data on whether oxygen strategies impact time-dependent harms related to invasive mechanical ventilation are lacking. A recent large meta-analysis across 21 RCTs found no significant differences in short-term, 90- or 180-day mortality, ICU-LOS, or ventilator use between conservative and liberal oxygenation strategies.10 However, despite the robust nature of this analysis and others like it, this analysis did not consolidate the most recent and large-scale RCTs with detailed patient outcomes like VFD-28.10
Objectives
This study seeks to determine the effect that liberal versus conservative arterial oxygen targets will have on the primary outcome of VFD-28, and secondary outcomes of all-cause mortality, and ICU LOS in adult patients receiving IMV.
Materials and Methods
Eligibility Criteria
Studies were eligible for inclusion if they enrolled adults (≥18 years) receiving invasive mechanical ventilation. Eligible interventions included liberal oxygenation strategies, defined as SpO₂ ≥96% or PaO₂ >100 mmHg, compared against conservative strategies, defined as SpO₂ 88–96% or PaO₂ 55–80 mmHg. To be considered, trials needed to report at least one of the following outcomes: 28-day ventilator-free days (VFD-28), all-cause mortality, or ICU length of stay (LOS). Only randomized controlled trials published between January 1, 1990 and June 26, 2025 were considered. Articles were restricted to the English language, although preprint manuscripts and conference abstracts were accepted if sufficient outcome data were available.
Information Sources and Search Strategy
A comprehensive search of the following electronic databases and trial registries was conducted from January 1, 1990 through June 26, 2025: MEDLINE via PubMed, Embase via Ovid, ClinicalTrials.gov, and WHO International Clinical Trials Registry Platform (ICTRP). All database searches combined the following key concepts with both controlled vocabulary (MeSH or Emtree) and free-text terms: (“oxygenation” OR “oxygen therapy” OR hyperoxia OR hypoxia) AND (“mechanical ventilation” OR ventilated OR “invasive ventilation”) AND (“randomized controlled trial” OR RCT OR randomised). Search filters were applied to restrict results to studies involving human adults (≥18 years) and English-language articles. Full search strings are provided in Appendix 1.
Selection Process
After application of automated screening tools, a single reviewer independently screened all remaining articles by title and abstract, eliminating those that did not meet inclusion criteria. The remaining articles were subsequently screened by reviewing their full-text versions. A list of automation tools used in screening is supplied in Appendix 2.
Data Collection Process
A single reviewer manually extracted relevant data from the full-text articles identified during the selection process. Contact with authors was not required, as all relevant data were extractable from published reports or supplementary materials.
Data Items
Data were extracted at three levels. At the study level, information including year of publication, country, study design (parallel-group, cluster-randomized, or cluster-crossover), sample size, and number of participating centers was collected. At the participant and intervention level, variables including mean or median age, sex distribution, baseline severity scores (APACHE II, SOFA), primary admission diagnoses, and achieved oxygenation targets (SpO₂ and PaO₂) were extracted. At the outcome level, data for VFD-28, all-cause mortality at multiple time points (ICU discharge, 28-day, 90-day, hospital discharge), and ICU LOS were collected. When outcomes were reported as medians with interquartile ranges (IQR), these were converted to means ± standard deviations (SD) using the Wan quantile-estimation method for samples ≥25 or the Hozo method for smaller samples. For cluster-randomized or cluster-crossover trials, we applied a design-effect adjustment to account for within-cluster correlation, using an intracluster correlation coefficient (ICC) of 0.01 and the reported or estimated average cluster size.
Outcome Prioritization
We prespecified 90-day all-cause mortality as the primary mortality time point to align with contemporary critical care trial standards and maximize clinical relevance. When 90-day mortality was not reported, we used the longest available follow-up (hospital discharge, 28-day, or ICU mortality) in sensitivity analyses. VFD-28 was defined as the number of days alive and free from mechanical ventilation through day 28; patients who died before day 28 were assigned 0 VFD. ICU LOS was analyzed as reported in each trial.
Risk of Bias Assessment
Risk of bias was assessed using the Cochrane Risk-of-Bias 2.0 tool, which evaluates five domains: randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selective reporting of results. Each domain was rated as “low risk,” “some concerns,” or “high risk,” and an overall judgment was assigned for each trial.
Effect Measures
For continuous outcomes (VFD-28 and ICU LOS), the mean difference (MD) was calculated as Conservative minus Liberal; negative values favor liberal oxygenation (more VFD or shorter LOS in the liberal arm). For all-cause mortality, risk ratio (RR) was used; RR >1 indicates higher mortality in the conservative arm.
Synthesis Methods
Random-effects meta-analyses were performed using the DerSimonian–Laird method-of-moments estimator for between-study variance (τ²). Heterogeneity was quantified using τ², I², and Cochran’s Q. For each outcome, we calculated 95% prediction intervals to estimate the range of effects expected in future similar trials.
For cluster-randomized or cluster-crossover trials (e.g., PILOT), we adjusted the effective sample size and variance using a design effect calculated as 1 + (m − 1) × ICC, where m is the average cluster size and ICC is the intracluster correlation coefficient (assumed to be 0.01 in primary analyses, with sensitivity analyses using ICC = 0.005 and 0.02).
When trials reported only medians and IQRs, we estimated means and SDs using the Wan method (for n ≥25) or Hozo method (for n <25). Sensitivity analyses restricted to trials reporting means and SDs directly were performed to assess the robustness of conversion methods.
Additional sensitivity analyses included:
-
Hartung–Knapp adjustment to account for uncertainty in τ² estimation
-
Leave-one-out analyses to assess influence of individual trials
-
Restriction to trials with low risk of bias
-
Alternative ICC assumptions for cluster-adjusted trials (0.005, 0.02)
All analyses were conducted in R version 4.x using the metafor package. Forest plots were generated with 95% confidence intervals, prediction intervals, and heterogeneity statistics (τ², I²).
Results
Study Selection
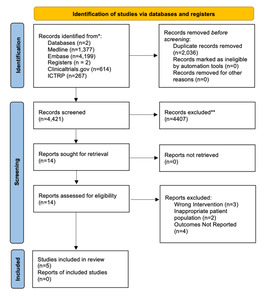
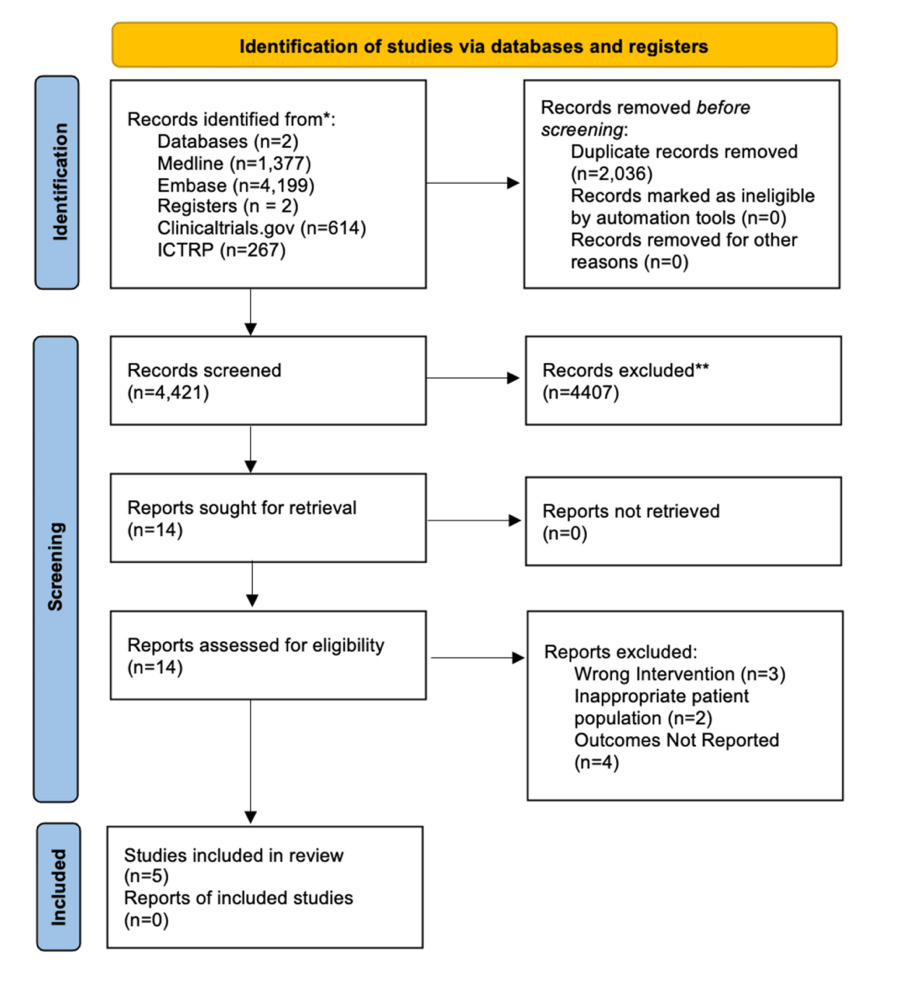
PRISMA 2020 study selection. We identified 6,457 records from MEDLINE (1,377), Embase (4,199), ClinicalTrials.gov (614), and WHO ICTRP (267). After removing 2,036 duplicates, 4,421 titles/abstracts were screened. Fourteen full texts were reviewed; nine were excluded (wrong intervention, n=3; inappropriate population, n=2; outcomes not reported, n=4). Five studies met criteria and were included in the synthesis.
Study Characteristics
The CLOSE trial (2016) was a multicenter RCT enrolling 103 adult patients who had received invasive mechanical ventilation for more than 24 hours.11 Patients were assigned to either a conservative oxygenation strategy targeting SpO₂ of 88-92% or a liberal strategy maintaining SpO₂ ≥96%.11 The primary outcome was 90-day ICU mortality, with secondary outcomes including organ dysfunction, VFD-28, and ICU LOS.11 The investigators found no statistically significant differences in mortality, organ dysfunction, ventilator-free days, or ICU LOS between the groups.11
The ICU-ROX trial (2019) was a multicenter, parallel-group randomized controlled trial (RCT) that compared conservative versus usual-care oxygenation strategies in critically ill adults.12 Patients were eligible if they were aged 18 years or older and receiving invasive or noninvasive mechanical ventilation for less than two hours.12 A total of 1,000 patients were randomized using a secure, centralized, internet-based system with computer-generated allocation, although clinicians were unblinded.12 The conservative strategy targeted SpO₂ values between 90-97%, while the usual-care group had no explicit upper SpO₂ limit.12 The primary outcome was ventilator-free days at 28 days (VFD-28), while secondary outcomes included all-cause mortality at 90 and 180 days, survival duration, return-to-work status at day 180, and cognitive and health-related quality of life assessed using the TICS and EQ-5D-5L questionnaires.12 The trial reported no significant differences between groups in VFD-28 or ICU length of stay (LOS).12
The PILOT trial (2022) employed a cluster-crossover design and randomized 2,541 mechanically ventilated ICU patients to lower, intermediate, or higher oxygenation targets.13 The lower group aimed for SpO₂ 90% or 88-92%, the intermediate group 94% or 92-96%, and the higher group 98% or 96-100%.13 The primary outcome was VFD-28, with additional analyses of mortality and ICU LOS. No significant differences were observed across groups in ventilator-free days, mortality, or ICU LOS.13
The ICONIC trial (2023) was a multicenter, parallel-group RCT that randomized 664 adult ICU patients expected to require at least 24 hours of invasive mechanical ventilation.14 The conservative group was managed with a PaO₂ target of 55-80 mmHg or SpO₂ 91-94%, while the liberal group targeted a PaO₂ of 110-150 mmHg or SpO₂ 96-100%.14 The primary outcome was 28-day all-cause mortality, with secondary outcomes including ventilator-free days and ICU LOS. Mortality was 38.5% in the low-oxygenation group compared with 34.7% in the high-oxygenation group, a difference that did not reach statistical significance.14 Similarly, no significant differences were observed in VFD-28 or ICU LOS.14
Finally, the UK-ROX trial (2025) was a large multicenter RCT that enrolled 16,500 mechanically ventilated adults in the ICU.15 Patients were randomized to a conservative oxygenation strategy, targeting SpO₂ of 90% (88-92%), or usual care, which typically involved aiming for SpO₂ ≥96% without a specified upper limit.15 The primary endpoint was 90-day all-cause mortality. Mortality was 35.4% in the conservative group and 34.9% in the usual-care group, with a risk difference of 0.7%.15 This difference was not statistically significant, and there was also no difference in ICU LOS between groups.15
Risk of Bias Assessment
The CLOSE trial (2016) was judged to have some concerns for risk of bias. Randomization was appropriately performed using a computer-generated sequence with opaque envelopes, and only 1.1% of the primary outcome data were missing.11 However, adherence to protocol was 82%, raising concerns about deviations from intended interventions.11 Outcome measurement also raised minor concerns because VFD and LOS were abstracted from electronic health records by unblinded assessors.11 Selective reporting was not identified, as the statistical analysis plan (SAP) had been preregistered and all outcomes were reported.11 Overall, the trial was considered to have some concerns of bias. In contrast, ICU-ROX (2019) was judged to be at low risk of bias. Central web-based randomization was used, separation of SpO₂ between groups exceeded 95%, and fewer than 1% of VFD data were missing.12 Outcomes were measured automatically, and both the protocol and SAP were published and concordant with reported outcomes.12 Similarly to the CLOSE trial, The PILOT trial (2022) was assessed as having some concerns for bias. Although randomization was robustly performed through a cluster-crossover design with balanced order checks, unblinded staff created the potential for co-intervention.13 Moreover, the observed SpO₂ separation between groups was only 3.1 percentage points.13 Missing outcome data were minimal (2.4%), outcome measurement relied on electronic collection with blinded statisticians, and selective reporting was not identified.13 The overall risk of bias was judged as some concerns, mainly due to deviations from intended interventions.13 Next, the ICONIC trial (2023) was judged to have low risk of bias. Randomization was performed by block randomization with sealed envelopes, achieving balanced baseline characteristics.14 PaO₂ separation between groups exceeded 45 mmHg, missing outcome data were limited to 1.5%, and outcome measurement was based on daily arterial blood gases.14 The registry and SAP were concordant with the published results, and no selective reporting was identified.14 Finally, UK-ROX (2025) was judged to have some concerns for bias.15 Randomization was centralized, stratified by site, and concealed, but protocol deviations occurred in 7% of FiO₂ assignments, and staff were unblinded.15 Although mortality data were complete, 2% of ICU LOS data were pending at the time of reporting, and the full dataset was only available in preprint form.15 Outcomes were extracted from the NHS database, which was considered reliable, and all prespecified SAP outcomes were presented.15 Overall, the trial was judged to carry some concerns of bias due to incomplete data release and modest protocol deviations.15
Outcomes
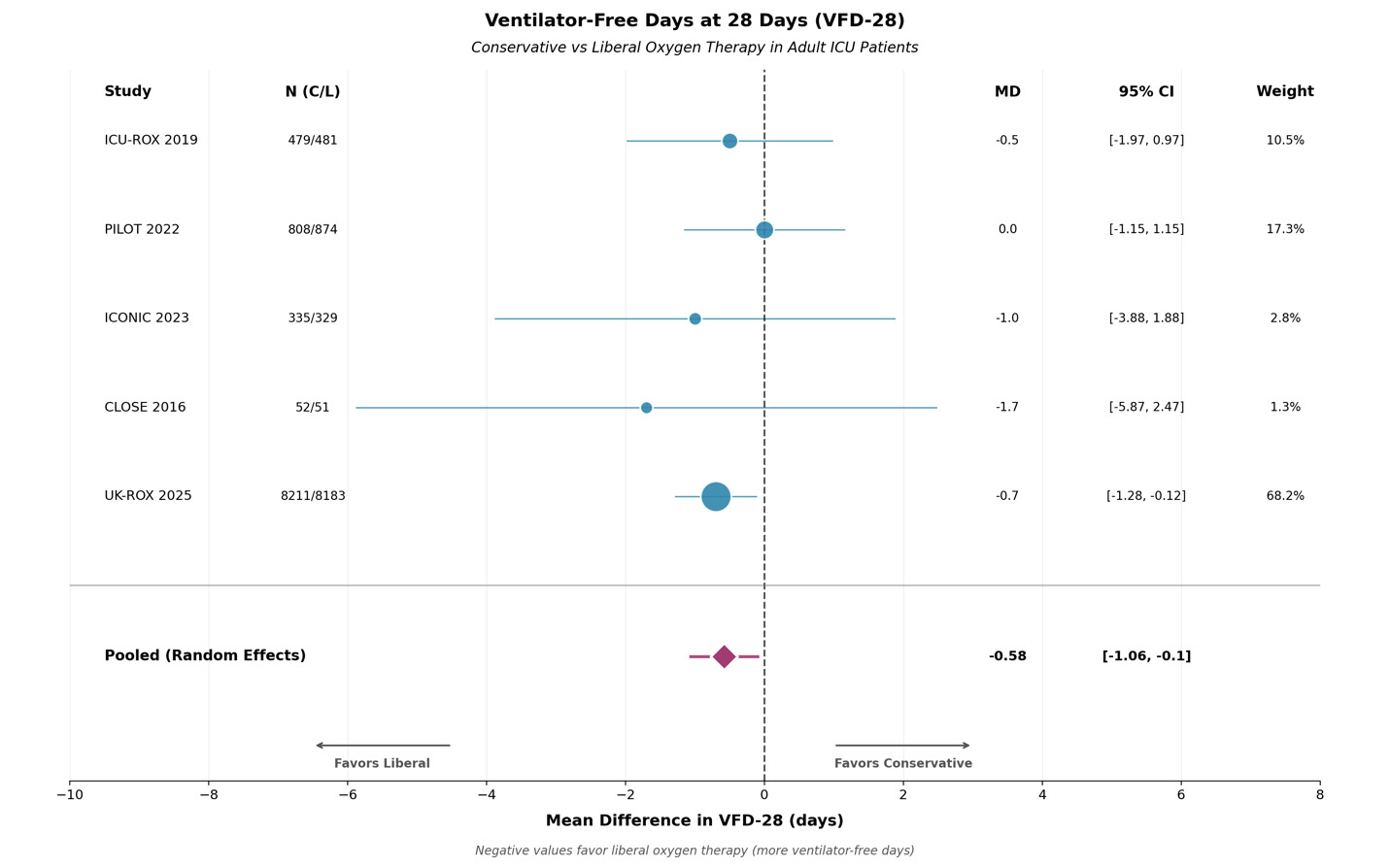
The primary outcome of interest in this study was VFD-28, and all five trials reported data on this outcome. Medians and SD were converted to means and SD using the Wan method. Means and SD were used and the mean difference of liberal and conservative oxygen groups was pooled using the REML model. This data suggests that liberal oxygen strategies increased ventilator free time by 0.42 days or 10 hours (MD -0.58, 95 % CI -1.06, -0.1; p = 0.017) when compared to conservative oxygen targets. There is no significant heterogeneity (I² = 0 %). An identical CI was produced by Hartung-Knapp adjustment. Leave-one-out analysis confirmed that no single trial significantly influenced the result. These results are summarized in Figure 2.
All five trials reported data on all-cause mortality. The pooled risk ratio (liberal vs conservative) was 1.022 (95 % CI 0.98–1.06; p = 0.266) under REML. Because the CI crosses unity, this result is statistically insignificant, indicating little-to-no survival benefit between the two strategies. A total of 3,509/9,882 (35.5%) patients died in the conservative group compared to 3,457/9,922 (34.8%) in the liberal group. The UK-ROX trial contributed 82.5% of the weight to this analysis, with no evidence of statistical heterogeneity. These results are summarized in Figure 3.
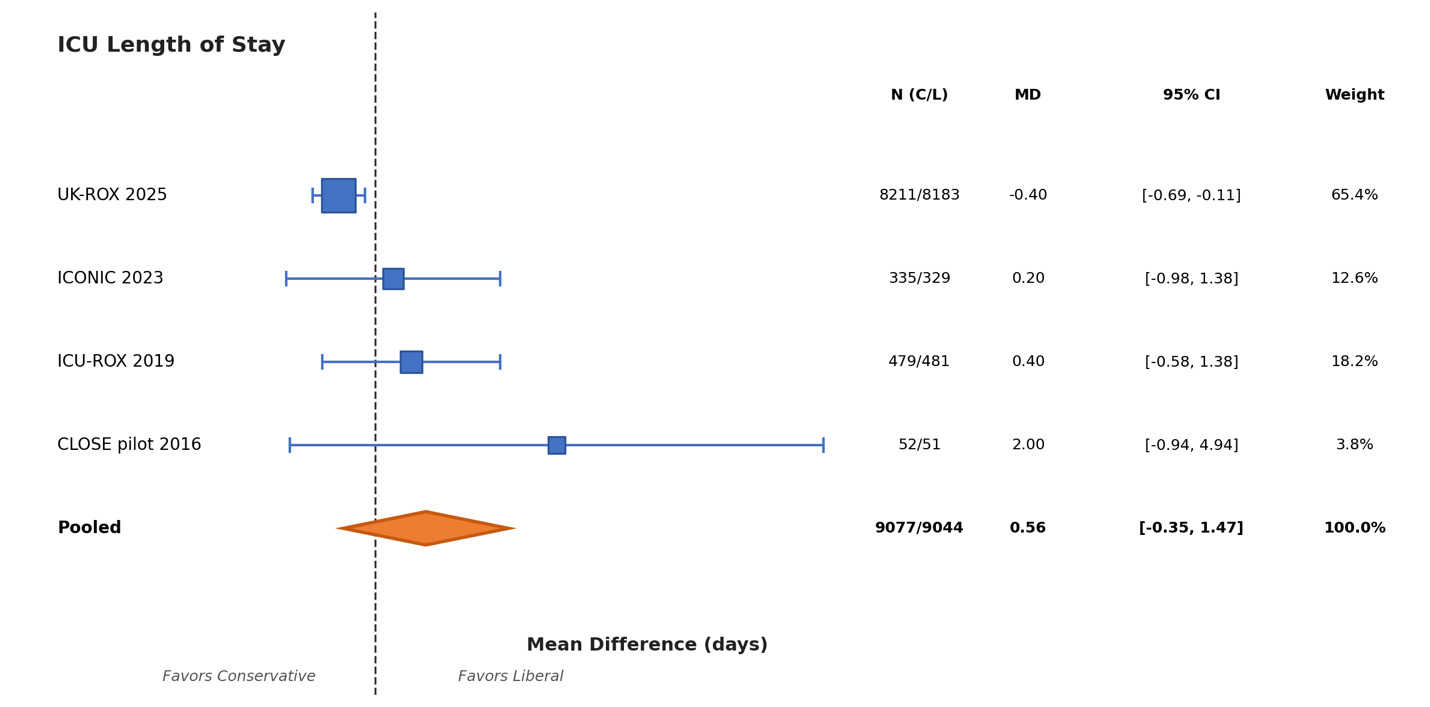
As far as ICU LOS was concerned, UK-ROX was the only trial that did not report ICU LOS. The pooled mean difference was 0.56 day (95 % CI —0.35 to 1.47; p = 0.227; I² = 82%) with high heterogeneity. These results are summarized in Figure 4.
Heterogeneity and Consistency
Statistical heterogeneity was absent for VFD-28 (I² = 0%, τ² = 0) and 90-day mortality (I² = 0%), indicating consistent treatment effects across trials for these outcomes. However, substantial heterogeneity was observed for ICU length of stay (I² = 82%, τ² = 0.64), suggesting variability in this outcome across the included studies.
Sensitivity Analyses
Multiple sensitivity analyses were performed to assess robustness. Leave-one-out analyses demonstrated stability of findings, with pooled mean differences for VFD-28 ranging from 0.63 to 0.69 days, all confidence intervals excluding zero. Mortality estimates remained stable (RR 1.01–1.03), though confidence intervals crossed unity, and ICU LOS results varied modestly (-0.14–0.42 days), with all confidence intervals crossing zero.
Fixed-effects analyses yielded results nearly identical to those of the random-effects model for VFD-28 and mortality (VFD-28 MD 0.65 days; mortality RR 1.02), supporting the validity of the random-effects framework. However, this was not the case for ICU-LOS
In contrast, ICU length of stay findings showed substantial sensitivity to analytical choices. Leave-one-out analyses demonstrated variability in pooled estimates ranging from -0.14 to 0.42 days, with the direction of effect changing based on study inclusion. Fixed-effects analysis yielded MD -0.29 days (95% CI -0.56 to -0.01), differing substantially from the random-effects estimate of 0.56 days (95% CI -0.35 to 1.47).
Overall, these sensitivity analyses confirmed the robust nature of our findings. Our results for VFD-28 and mortality were not driven or influenced by any study, choice of model or heterogeneity estimator. However, the ICU-LOS data demonstrated high heterogeneity (I² = 82%) and should be interpreted with caution. Leave-one-out analyses showed substantial variability (MD range -0.14 to 0.42 days), with direction of effect changing based on study inclusion. Fixed-effects analysis (MD -0.29 days, 95% CI -0.56 to -0.01) differed substantially from random-effects estimates (MD 0.56 days, 95% CI -0.35 to 1.47), suggesting the pooled ICU-LOS estimate lacks robustness.
Reporting Bias Assessment
Egger’s regression tests yielded non-significant results for mortality (p = 0.83), borderline results for VFD-28 (p = 0.05), and significant small-study effects for ICU-LOS (p = 0.012). The significant Egger test for ICU-LOS, combined with high heterogeneity (I² = 82%), suggests caution in interpreting the pooled estimate. Funnel plot asymmetry for ICU-LOS may reflect genuine between-study heterogeneity rather than publication bias, given that all trials were prospectively registered and reported prespecified outcomes.
Certainty Assessment
VFD-28 was determined to have moderate certainty. This level of certainty was assigned to this variable due to concerns regarding imprecision due to the small absolute mean difference and relatively narrow confidence interval approaching null. Similarly, all-cause mortality was determined to have a moderate level of certainty. This variable was primarily downgraded due to concerns regarding imprecision. Imprecision is a concern due to the pooled risk ratio crossing the line of no effect. Finally, ICU-LOS was determined to have a low level of certainty. This variable was downgraded due to concerns of imprecision, inconsistency and possible publication bias. The pooled mean difference of 0.56 indicates a possible benefit, but the CI crosses zero (-0.35 to 1.47), indicating a lack of statistical significance and include both harm and benefit. In addition, there was heterogeneity between studies (I² = 82%). Finally, there is a concern for possible publication bias due a significant Egger’s test (p=0.012). For these reasons, there was a two-level downgrade for this variable.
Prediction Intervals
To contextualize uncertainty, 95% prediction intervals (PIs) were calculated. For VFD-28, the PI ranged from –1.21 to -0.16 days, suggesting that future trials might observe either a slight disadvantage or a modest benefit from liberal oxygenation, despite the pooled average benefit of -0.58 days. For mortality, the PI was 0.78–1.32. For ICU LOS, the PI ranged from –1.20 to 1.96 days, implying that liberal oxygen could either prolong ICU stay by over a day or shorten it by nearly two days. These wide intervals underscore the uncertainty and context-specific variability of oxygenation strategies across patient populations.
Discussion
Summary of Main Findings
In a random-effects meta-analysis of 5 separate trials (n≈19,403), liberal oxygen targets were associated with a modest benefit in VFD-28 (MD -0.58, 95 % CI -1.06, -0.1, I² = 0 %). There was no substantial effect on mortality (RR 1.02, 95 % CI 0.98–1.06; I² = 0%) or ICU LOS (MD 0.56 days, 95 % CI –0.35, 1.47; I² = 24 %). Prediction intervals showed a wide variety of anticipated future findings, ranging from slight harm to modest benefit. In summary, liberal oxygen-target strategies demonstrate a very modest benefit when compared to more conservative oxygen targets, with no clear effects on mortality and ICU length-of-stay.
Strengths and Limitations of the Evidence
The strengths of this study are numerous. Firstly, the inclusion of UK-ROX which is a recent large RCT (n = 16,400) is a major advantage of this study. In addition, this study used patient centered outcomes that are directly correlated to morbidity and mortality such as VFD-28, ICU-LOS and all-cause mortality. Finally, this study utilized robust random effects modeling through the use of REML with Hartung-Knapp CIs, heterogeneity analyses, prediction intervals and sensitivity analyses.
Despite the strengths of this study, its limitations are also numerous. The primary limitation in this study stems from the forced reliance on aggregate data. This can manifest in a few ways. Firstly, the conversions of medians to means could have skewed ICU LOS distributions. Furthermore, the classification of liberal and conservative oxygen strategies varied between groups. Finally, adverse events such as re-intubation rates, severe hypoxemia, and others were not uniformly reported which prevented pooled analyses.
Limitations of the Review Process
Despite the comprehensive and exhaustive search for available trial data using MEDLINE and Embase, data extraction and screening were performed by a single reviewer. In addition, only articles written in the English language were included which could have inadvertently narrowed down the data pool.
Implications for Practice, Policy and Research
Even though there was a statistically significant, albeit marginal, increase in the ventilator free days in the liberal oxygenation group clinicians must consider whether this marginal gain is worth the potential adverse effects of hyperoxemia-oxidative stress and cellular injury,1,2 absorption atelectasis,16 pulmonary toxicity,17 reduced cardiac output,18 endothelial dysfunction19 and increased mortality [22]. In our pooled analysis, these adverse events did not translate into higher observed mortality or ICU LOS stays, but individual patient susceptibility varies. Therefore, clinicians should individualize oxygen targets, balancing the modest gains against each patient’s unique risk profile. Because of this, some institutions might consider utilizing a mid-range “safe-zone” with an SpO2 ranging between 92-96%. This is in contrast to strict conservative or liberal strategies. Finally, significant uncertainty still exists in regards to the frequency and severity of adverse events associated with each oxygen strategy. Therefore, future research should be conducted on the following topics: Quantifying rates of adverse events associated with each oxygen target, identification of specific subgroups that might benefit from one strategy over another, and evaluating long-term outcomes and quality of life scores after hospital discharge.
Conclusion
In all adult ICU patients, there was a modest increase in the amount of ventilator free days that did not affect ICU LOS or all-cause mortality. However, this benefit is very small, and the theoretical potential for adverse events related to both hyperoxia and hypoxia still exist. Therefore, an individualized oxygen-target strategy should be used for each patient, balancing the aim of avoiding hypoxemia against each patient’s susceptibility to hyperoxemia and oxygen-related toxicity, while facilitating earlier ventilator liberation. Oxygen goals should be applied dynamically as ventilatory support, sedation, and hemodynamics change. The aim is steady, safe oxygenation, not rigid adherence to a single number. Future work should identify which patients truly benefit from liberal versus conservative targets.
Declaration of Funding
No funding was received.
Corresponding author
Christopher Fay, DO
Department of Graduate Medical Education
Morehouse School of Medicine
720 Westview Drive SW,
Atlanta, GA 30310, USA
Phone: (404) 518 3332
Email: cfay@msm.edu
Author Contributions
CF is the sole author of this manuscript. CF was responsible for all steps in the generation of this manuscript including: literature review, statistical calculations, sensitivity analyses, coding in Rstudio, manuscript writing, manuscript reviewing, final editing and manuscript submission.
Disclosures
There are no conflicts of interest to disclose.