Introduction
Cricothyrotomy is a rare but life-saving intervention reserved for “cannot intubate/cannot ventilate” scenarios. Because it is often a last-resort procedure, opportunities for real-world practice are limited.1 Consequently, physician preparedness remains highly variable. In one survey of emergency medicine physicians, only 22% reported performing a cricothyrotomy during residency, and just 51.6% had ever witnessed one being performed during residency.2 This low frequency underscores the importance of simulation-based training for skill acquisition and retention. Repetitive practice with accessible trainers reinforces muscle memory and helps maintain procedural competence outside the clinical environment.
Existing high-fidelity models, however, are prohibitively expensive. Prior studies suggest that low-fidelity models may bear similar efficacy to high-fidelity models.3 Massoth et al. demonstrated that medical students trained on low-fidelity trainers performed comparably to peers trained on high-fidelity models when later tested on cadavers.3 These findings support the role of low-cost trainers for repetitive practice and skill maintenance. Building on this evidence, the present trainer aims to provide a practical, affordable, and anatomically realistic tool that can be used in home or wet-lab settings to help providers maintain proficiency in this critical procedure. To further guide its development, a qualitative literature review was conducted to evaluate previously published trainers and identify existing gaps in the field.
Methods
Building the Model
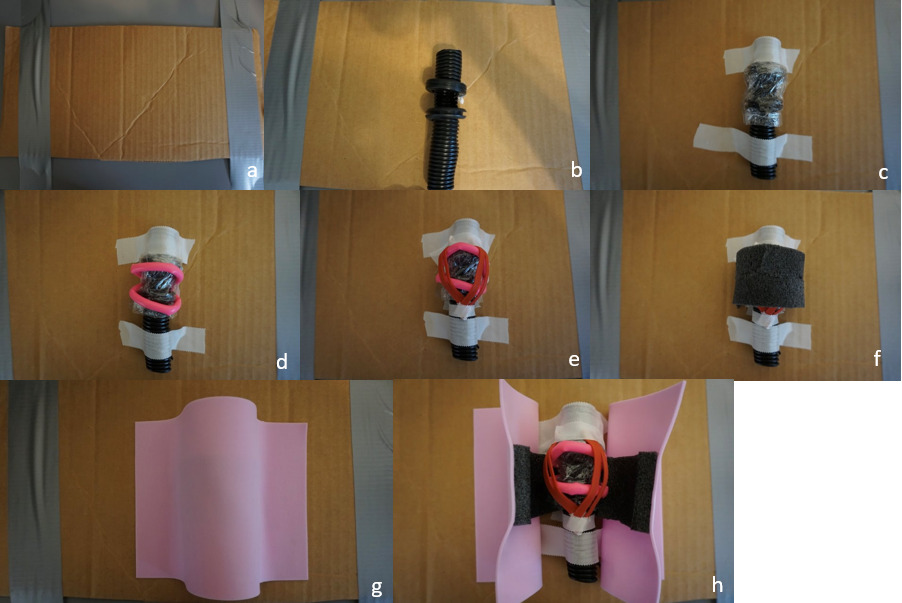
Eight steps (with corresponding images) were created to build the proposed model:
-
Prepare the base (Image 1a):
With a utility knife, cut a 6 x 8-inch section from a cardboard box to serve as the base for the model. Place the base on a flat working surface.
-
Construct the trachea (Image 1b):
Cut a 4-5 inch length of black corrugated wire tubing. On one side, cut a rectangular window approximately 1-2 cm high x 2-3 cm wide to represent the anterior tracheal opening.
-
Prepare the cricoid ring (Image 1b):
Cut a wedge out of 2 rubber grommets to create openings. Remove approximately 1 cm of material from the grommets. This will allow the grommets to fit snugly over the tubing to simulate the cricoid and thyroid cartilage.
-
Simulate the cricothyroid membrane (Image 1c):
Wrap the tubing and attached grommets tightly with plastic wrap. The wrap should be taught to provide realistic resistance when cut, simulating the cricothyroid membrane. Position the tube in the center of the cardboard base and fasten it securely using adhesive tape, ensuring it will not shift during simulated procedures.
-
Add vascular structures (Image 1d):
Fill a modeling balloon with lotion using a syringe, then tie it off securely. Attach the lotion-filled balloon superior and inferior to the grommets to represent vessels in proximity to the airway. Secure in place with adhesive tape.
-
Add the cricothyroid muscles (Image 1e):
Cut two rubber bands in half. Arrange the four halves into a “V” formation over the grommet and balloon to mimic the cricothyroid muscles. Secure with adhesive tape.
-
Simulate subcutaneous tissue (Image 1f):
Cut a 3-inch segment from the foam pipe insulation. Split it lengthwise and trim thickness as desired to represent varying amounts of subcutaneous fat. Position this layer over the muscle and vessel structures.
-
Add skin layer (Image 1g):
Place a single adhesive foam sheet over the entire construct to represent skin. Ensure the edges are flush with the model.
Conducting the Literature Review
A systematic literature review was conducted using publicly available academic databases and search engines, including PubMed (PubMed, n.d.) and Google Scholar (Google Scholar, n.d.). Studies were eligible for inclusion if they (1) explicitly self-identified their model as a low-cost and/or low-fidelity cricothyrotomy trainer within the title or manuscript, and (2) provided photographic documentation and/or written instructions with a materials list for model construction.
Each trainer was evaluated against seven predefined criteria:
-
Use of 3D-printed materials
-
Use of biologic materials (i.e. porcine or cadaveric skin)
-
Initial cost (≤40 USD vs. >40 USD)
-
Reuse cost (≤1 USD vs. >1 USD per use)
-
Setup time (≤20 minutes vs. >20 minutes)
-
Incorporation of mannequins or artificial skin
-
Accessibility of materials (availability of components including: 3D-printed parts, biologic specimens, mannequins, or artificial skin).
Criteria related to materials (3D printing, biologic tissues, mannequins, or artificial skin) were assessed based on the manuscript’s build instructions and/or accompanying photographic documentation. Cost and setup time were categorized dichotomously, as noted above.
Results
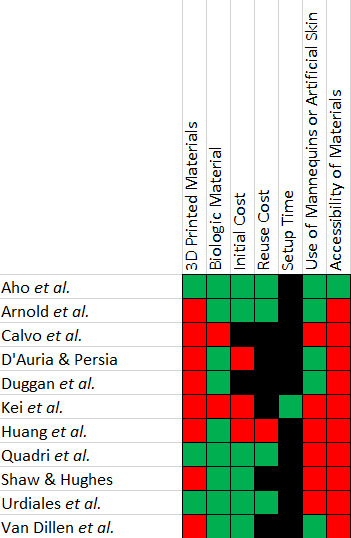
The novel cricothyrotomy trainer met six of the seven predefined criteria. Total material costs were $34, inclusive of reuse costs for approximately 30 subsequent attempts, as supplies were purchased in bulk. Itemized pricing and sources are presented in Table 1. based on the online site listings at the time of purchase in August 2025. Eleven articles met the inclusion criteria for review and were assessed using the seven predefined factors (Table 2).4–14 Eight of eleven studies (73%) incorporated 3D-printed components as their primary structural element. Two studies (18%) utilized biologic materials, specifically porcine skin. Nine studies reported the initial cost. Of these, three (33%) required an investment greater than $40, while six (67%) described models with a value below this threshold. Reuse cost was reported in five studies; one exceeded $1.00 per use, while four were ≤$1.00. Setup time was documented in one study, reported as less than one minute. Six studies (55%) incorporated mannequins or artificial skin. Ten of eleven studies (91%) used materials not widely available to individual practitioners, including proprietary mannequins, specialized artificial tissues, or custom 3D-printed parts.
This table illustrates the assembly cost for the cricothyrotomy trainer. This is the total “upfront” cost, which includes the cost for approximately 30 subsequent attempts. The listed prices were recorded at the time of purchase in August 2025 from online site listings and local department store prices.
The eleven included manuscripts were evaluated with seven predefined factors. Each factor was dichotomized as either present/absent, or as above/below a defined threshold. Green boxes indicate favorable characteristics (no use of 3D-printed or biologic materials, initial cost < $40, reuse cost < $1.00, setup time < 20 minutes, no use of artificial skin or mannequins, and reliance on readily accessible materials). Red boxes indicate the presence of 3D-printed or biologic materials, the use of mannequins/artificial skin, costs exceeding the threshold for initial or reuse costs, or setup times exceeding 20 minutes. Black boxes indicate that the corresponding criterion was not reported in the article.
Discussion
Simulation is crucial for enhancing efficiency and developing muscle memory in procedural practice. Traditionally, high-fidelity trainers have been considered the gold standard; however, these models can cost $400–$1,500, creating barriers to repetitive practice for individual providers.14 Furthermore, several studies have challenged the superiority of high-fidelity models. Lee et al., Matsumoto et al., and de Giovanni et al. demonstrated the non-inferiority of low-fidelity simulations when compared to their high-fidelity counterparts.15–17 These findings suggest that muscle memory formation and repetition are more critical to skill acquisition than the fidelity of the simulator.
Cricothyrotomy is a rare but life-saving procedure required in emergency medicine, otorhinolaryngology, and paramedicine. Maintaining proficiency is essential, yet opportunities for practice are limited. A simple, inexpensive, and widely accessible trainer may therefore serve as a sufficient medium for practice. With this goal in mind, we designed a model using only low-cost materials available in large department stores. The model successfully met its predetermined financial goals, and all materials were sourced from widely available chain stores.
According to our literature review, 73% (8/11) of the reviewed models incorporated 3D-printed components, most commonly for constructing tracheas. However, 3D printing presents accessibility challenges: printers cost $200–$300, which may be prohibitive for individual practitioners or institutions without existing infrastructure.10 To overcome this limitation, we utilized 20 mm corrugated tubing. 20 mm lies within the diametric range of an average adult male trachea.18
Another limitation observed in the reviewed models was oversimplification. Minimal-part designs, such as those by Shaw & Hughes, allow rapid construction and classroom scalability but sacrifice anatomical fidelity.12 Notably, several models7,8,10 lacked a distinct cricothyroid membrane. This omission eliminates the tactile feedback—the characteristic “pop” as the scalpel passes through the membrane—that is a critical learning point during cricothyrotomy. This model aims to address the lack of anatomical detail by incorporating additional features to provide a more accurate representation, thereby striking a balance between efficiency and accuracy.
A primary limitation of maximizing individual anatomical components is the increased setup time. In contrast to models such as those by Shaw & Hughes and Huang et al., which prioritize efficiency by using minimal parts, our design requires a greater initial time investment to construct each structure.10,12 However, once the reusable components—including strap muscles, trachea, and cartilages—are assembled, subsequent setup time is minimal (<2 minutes). The rationale for incorporating distinct anatomical parts is to encourage deliberate palpation of critical structures, including adipose tissue, vascular landmarks, and cartilages, thereby promoting a more realistic and mindful training experience. A second limitation is the reliance on store-bought materials rather than higher-fidelity options such as porcine skin or mannequins. While this results in a less realistic experience, the intent of this trainer is to facilitate repeated practice rather than maximize fidelity.
Conclusion
Following a review of the literature, an apparent gap was identified in low-fidelity cricothyrotomy trainers. Specifically, a large majority of cricothyrotomy models were built with 3D printed materials. While a useful and practical medium, not all practitioners have access to a 3D printer. Additionally, many models use sparingly few parts, which minimizes setup time but oversimplifies anatomy. This model addressed these problems by using only widely available materials and aimed to create individual, unique anatomical structures with craft and hardware supplies. Our model not only addresses an unmet educational need but also empowers institutions and learners with a low-barrier, high-value tool that can be integrated into simulation curricula as early as the MS-1 and MS-2 levels. Because it is inexpensive and easy to assemble, it lends itself to widespread adoption, even in resource-limited settings.