Introduction
Capecitabine, an oral prodrug of 5-fluorouracil (5-FU), is widely used in the treatment of colorectal and breast cancers, both in the adjuvant and metastatic settings.1 Although generally well tolerated, capecitabine is associated with common toxicities such as hand–foot syndrome, diarrhea, and myelosuppression. Rarely, cutaneous adverse events such as vasculitis may occur and pose significant diagnostic and therapeutic challenges.2–4
Leukocytoclastic vasculitis (LCV) is an immune complex–mediated small vessel vasculitis characterized by neutrophilic infiltration, fibrinoid necrosis, and leukocytoclasia.5 Clinically, it manifests as palpable purpura, typically on the lower extremities, and may be associated with infections, systemic autoimmune diseases, malignancy itself, or drug exposure.6,7
Although fluoropyrimidine-induced vasculitis is exceedingly rare, isolated cases have been reported with both 5-FU and capecitabine.2–4 Recent reviews have highlighted chemotherapy-induced vasculitis as an emerging but underrecognized adverse event, stressing the importance of early recognition to avoid unnecessary investigations or delays in treatment.8,9
Here, we report a rare case of capecitabine-induced leukocytoclastic vasculitis in a patient with colorectal cancer, underscoring the importance of prompt diagnosis and drug withdrawal to ensure optimal patient management.
Case Report
A 67-year-old man with a history of hypertension and type 2 diabetes mellitus was followed at the Department of Medical Oncology, Emile Durkheim Hospital, Épinal, France, for stage III colorectal adenocarcinoma. He underwent curative surgical resection and was subsequently started on adjuvant capecitabine monotherapy, as his comorbidities rendered him unfit for oxaliplatin-based chemotherapy.
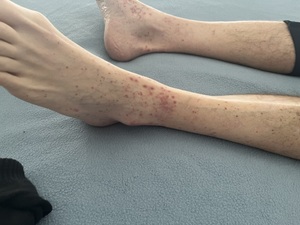
After the third cycle of capecitabine, he developed a purpuric rash predominantly affecting both lower extremities. Clinical examination revealed multiple palpable purpuric lesions symmetrically distributed on the legs and ankles, without ulceration, necrosis, or mucosal involvement. No systemic manifestations such as fever, arthralgia, abdominal pain, or renal symptoms were observed (Figure 1).
A closer view of the ankles showed erythematous and purpuric lesions consistent with leukocytoclastic vasculitis (Figure 2).
Laboratory investigations were performed to exclude alternative etiologies. Complete blood count was normal, with no thrombocytopenia or leukocytosis. Serum electrophoresis and immunoglobulin levels were within reference ranges. Autoimmune markers, including antinuclear antibodies (ANA), anti-dsDNA, anticardiolipin antibodies, and antineutrophil cytoplasmic antibodies (ANCA), were negative. Complement levels (C3, C4, CH50) were normal, and tests for cryoglobulins and circulating anticoagulants were unremarkable. Viral serologies, including hepatitis B, hepatitis C, cytomegalovirus, and parvovirus B19, were all negative.
The patient was not receiving any concomitant medications other than his usual antihypertensive and antidiabetic treatments at the time of capecitabine therapy, and had no additional relevant past medical history.
A skin biopsy was not performed due to the rapid improvement of lesions following discontinuation of capecitabine and initiation of corticosteroid therapy. The diagnosis was therefore established on clinical grounds, supported by the exclusion of alternative etiologies.
Capecitabine was discontinued, and the patient was managed with supportive care and oral corticosteroids. Over the following weeks, the cutaneous lesions progressively resolved, with no recurrence during subsequent follow-up.
Discussion
Drug-induced leukocytoclastic vasculitis (LCV) is a rare but important differential diagnosis in oncology patients presenting with new-onset purpura. Classically, LCV presents with palpable purpura on the lower extremities, as in our patient, but it may mimic other etiologies such as thrombocytopenia, infectious vasculitis, disseminated intravascular coagulation, or paraneoplastic syndromes.5–7
The gold standard for diagnosing LCV is a skin biopsy of a recent lesion, ideally within 24–48 hours of appearance, which typically demonstrates neutrophilic infiltration, fibrinoid necrosis, and leukocytoclasia.5,10,11 Direct immunofluorescence (DIF) may further help to identify immune complex deposits and guide toward specific etiologies.11 In our case, a skin biopsy was not performed due to the rapid improvement of lesions following discontinuation of capecitabine and initiation of corticosteroid therapy. The diagnosis was therefore established on clinical grounds, supported by the exclusion of alternative etiologies. Importantly, the patient had no additional relevant past medical history and was not receiving any concomitant medications other than his usual antihypertensive and antidiabetic treatments, further reducing the likelihood of alternative drug-induced or systemic causes. While the absence of histopathological confirmation represents a limitation, the typical clinical presentation, absence of systemic involvement, negative laboratory work-up, and the temporal relationship with capecitabine initiation strongly supported the diagnosis.
Several recent reports have described capecitabine-induced LCV with variable presentations. Jallouli et al. reported one of the earliest cases in gastrointestinal oncology,2 followed by Ribeiro et al.4 and more recent observations.3 In a 2021 case, Dogan et al. confirmed the diagnosis histologically.3 More recently, Arici et al. (2024) described a case of capecitabine-induced LCV successfully managed with corticosteroids, in which the diagnosis was pathologically confirmed.12 These cases emphasize the importance of considering drug-induced vasculitis when evaluating new purpuric lesions in patients on chemotherapy.
Management of capecitabine-induced vasculitis relies primarily on drug withdrawal. Supportive care alone is sufficient in mild cases, while corticosteroids may be used in severe or extensive presentations.3,4,12 Rechallenge is generally avoided due to the risk of recurrence, though occasional reports describe cautious reintroduction under close monitoring.3
Recent systematic reviews have emphasized that chemotherapy- and targeted therapy–related vasculitis, although rare, may be underreported in oncology.8 Furthermore, updated analyses highlight the importance of multidisciplinary evaluation in suspected cases, as early recognition avoids unnecessary biopsies, extensive investigations, or inappropriate treatment interruptions.9,13
Our case illustrates the limitations of routine diagnostic work-up and emphasizes the importance of considering drug-induced vasculitis early in the evaluation of purpura in cancer patients. Oncologists should remain vigilant for this rare toxicity to ensure accurate diagnosis and timely management.
Conclusion
Drug-induced leukocytoclastic vasculitis is a rare but important differential diagnosis in oncology patients presenting with new-onset purpura. Classically, LCV presents with palpable purpura on the lower extremities, as in our patient, but it may mimic other etiologies such as thrombocytopenia, infectious vasculitis, disseminated intravascular coagulation, or paraneoplastic syndromes.5–7
In our case, the absence of systemic involvement, normal platelet count, negative infectious and immunological work-up, and the temporal relationship with capecitabine strongly supported a drug-induced mechanism. Resolution of the lesions after discontinuation of capecitabine further confirmed the diagnosis, consistent with previously reported cases.2–4
Management of capecitabine-induced vasculitis relies primarily on drug withdrawal. Supportive care alone is sufficient in mild cases, while corticosteroids may be used in severe or extensive presentations. Rechallenge is generally avoided due to the risk of recurrence, though occasional reports describe cautious reintroduction under close monitoring.2,3
Recent systematic reviews have emphasized that chemotherapy- and targeted therapy–related vasculitis, although rare, may be underreported in oncology.8 Furthermore, updated analyses highlight the importance of multidisciplinary evaluation in suspected cases, as early recognition avoids unnecessary biopsies, extensive investigations, or inappropriate treatment interruptions.9
Our case illustrates the limitations of routine diagnostic work-up and emphasizes the importance of considering drug-induced vasculitis early in the evaluation of purpura in cancer patients. Oncologists should remain vigilant for this rare toxicity to ensure accurate diagnosis and timely management.
Acknowledgements
The authors would like to thank the patient and his family for their trust and cooperation. We also acknowledge the medical and nursing staff of the Department of Medical Oncology, Emile Durkheim Hospital, Épinal, France, for their support in patient care and data collection.
Patient Consent
Written informed consent was obtained from the patient for publication of this case report and the accompanying clinical images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Conflict of Interest
The authors declare no conflict of interest related to this work.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Statement
This article describes a single patient case report. According to institutional policies, ethics committee approval was not required. Patient confidentiality was maintained throughout.
Data Availability
All data generated or analyzed during this study are included in this published article.