Introduction
Lewy Body Dementia (LBD) is one of the most common yet often overlooked neurodegenerative disorders in the world. It affects an estimated 1.4 million people in the United States alone.1,2 LBD consists of two closely related diagnoses: Dementia with Lewy Bodies (DLB) and Parkinson’s Disease Dementia (PDD). In DLB, cognitive symptoms happen before or within one year of the onset of parkinsonism. In PDD, dementia develops only after a long period of motor symptoms.3,4 Together, these conditions make up about 5 to 10% of all dementia cases in older adults. This classification makes LBD the third most common type of dementia, following Alzheimer’s disease and vascular dementia.2,5
LBD is classified as an alpha-synucleinopathy, a type of neurodegenerative disorder. It features the harmful buildup of misfolded alpha-synuclein protein in neurons.6 In LBD, Lewy bodies collect in the cortex, limbic structures, and brainstem. This accumulation interrupts cellular function. The condition has some similarities to Parkinson’s disease but also distinct clinical features. Early symptoms include visual hallucinations, delusions, cognitive fluctuations, REM sleep behavior disorder (RBD), and problems with autonomic functions.3,7,8
Although motor features, such as bradykinesia and rigidity, are often present, non-motor psychiatric and behavioral symptoms frequently take over the clinical course and greatly reduce quality of life.9 Cholinergic dysfunction in LBD is more severe than in Alzheimer’s disease, leading to hallucinations and problems with attention. Meanwhile, the loss of dopaminergic neurons causes parkinsonian features, making differential diagnosis more challenging.6,7
The average age of onset for LBD is around 70 years. Its progression puts a heavy burden on older adults and their caregivers. However, LBD is often underdiagnosed or misdiagnosed in about 50% of cases. This is due to symptom overlap with Alzheimer’s and Parkinson’s diseases, along with a lack of clear biomarkers.4,10 The revised DLB Consortium criteria attempt to tackle this issue by highlighting key features such as RBD and REM without atonia, as detected through polysomnography. They also include DAT-SPECT and myocardial MIBG imaging to improve diagnostic accuracy.3,11
The neuropsychiatric symptom spectrum and caregiver burden are a critical yet underexplored aspect of LBD. Confounding factors such as hallucinations, agitation, apathy, and depression can affect up to 80% of the people with LBD; moreover, these factors are strongly correlated with the early need for institutionalization, the costs of healthcare, and the distress of the caregiver.9,12 In contrast to the typical decline in Alzheimer’s disease that is noticed, LBD is marked by the fast development of symptoms and their fluctuation; hence, it often leaves caregivers unprepared. Not only this, elderly patients with LBD are usually afflicted by comorbidities, polypharmacy, and face the barrier of not getting the treatment—these things all together lead to the worsening of the disease and increase the burden of the caregiver.10,12
This bibliometric analysis covers literature published from 1986 to 2025, using the Web of Science Core Collection. The goal is to create a broad view of how the neuropsychiatric aspects of LBD and their effects on caregivers have been studied across different fields, regions, and over time.
Methods
Bibliometric analysis is a method that quantifies literature and distinguishes the publication trends, key papers and authors, and countries or institutions’ contributions within a particular field of research.13 Standard bibliometric methods for medical literature were followed to ensure rigor and reproducibility.14
The information for this analysis was retrieved from the Web of Science Core Collection, which was chosen for the breadth of indexed peer-reviewed articles and compatibility with the bibliometric visualization software that it offered. The search was conducted through the advanced search, limited to articles and review papers published between 1986 and June 1, 2025, while no language restrictions were applied. The retrieved publications were exported and analyzed using VOSviewer (Leiden University, Netherlands), a software tool for constructing and visualizing bibliometric networks.14
The exact search strategy was as follows:
TS=((“Lewy body dementia” OR “LBD” OR “dementia with Lewy bodies” OR “DLB” OR “alpha-synucleinopathy”) AND (“neuropsychiatric symptoms” OR “neuropsychiatric” OR “behavioral symptoms” OR “behavioral” OR “psychological symptoms” OR “psychological” OR “hallucinations” OR “delusions” OR “REM sleep” OR “RBD” OR “autonomic dysfunction” OR “sleep disturbances” OR “agitation” OR “aggression” OR “depression” OR “anxiety” OR “apathy” OR “paranoia” OR “insomnia”) AND (“caregiver” OR “carer” OR “caregiving” OR “care partner” OR “family member” OR “family caregiver” OR “informal caregiver” OR “spouse” OR “burden” OR “stress” OR “strain” OR “support” OR “psychosocial”))
Results
A total of 770 articles were received to analyze in VOSviewer. The set had a range of final publication years from 1986 to 2025. (Figure 1)
Since the first paper on Lewy Body Dementia (LBD) was published in 1986, research output has grown steadily, at first gradually, then more noticeably after 2020. That year, 56 relevant studies were indexed (Figure 1). By 2024, the number had jumped to 73, marking an increase of around 30%. So far in 2025, 26 papers have been published, though it’s still early in the year. This suggests a continued upward trend, though the full year’s total remains to be seen.
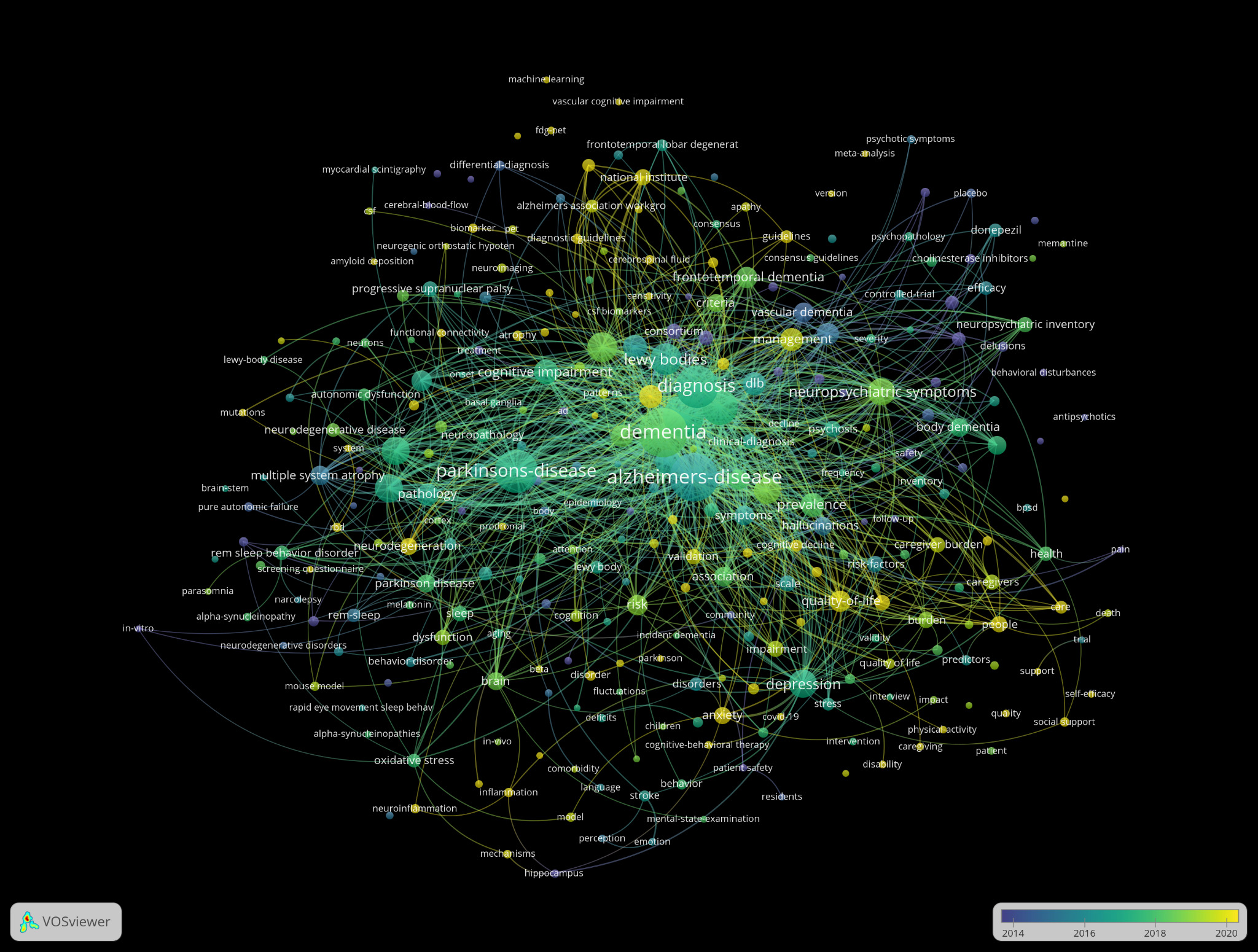
Surveying the evolution of language used in Lewy Body Dementia (LBD) research revealed clear temporal clustering in term usage within titles and abstracts (Figure 2). Earlier terms (shown in cooler colors) were concentrated around core pathological and preclinical concepts such as “alpha-synuclein,” “substantia nigra,” “mutation,” and “mouse,” reflecting an initial research emphasis on biological mechanisms. These terms also showed strong link strength, for example, “alpha-synuclein” had one of the highest total link strengths in the network, indicating deep integration across multiple research threads.
In contrast, newer terms such as “psychological symptoms,” “care partner,” and “neuropsychiatric inventory” appeared more recently and also demonstrated high relevance and connectivity, suggesting a robust shift toward behavioral symptoms and caregiving outcomes. For instance, “psychological symptoms” appeared 34 times and were closely linked to both clinical intervention terms (like “memantine”) and caregiver-centered concepts (like “BPSD” and “care partner”).
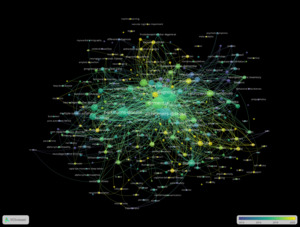
The most frequent terms in the author keywords network were “dementia with Lewy bodies” (171 occurrences), “dementia” (152), “Alzheimer’s disease” (135), and “Parkinson’s disease” (82), forming the central cluster of the network. These terms appeared with both high frequency and high relevance.
Additional terms such as “Lewy body dementia” (63), “Lewy body disease” (39), and “alpha-synuclein” (35) were also prominent. Neuropsychiatric and behavioral symptomatology was represented by keywords like “depression” (37), “hallucinations” (21), “REM sleep behavior disorder” (23), and “neuropsychiatric symptoms” (27).
The network structure showed tight clustering around dementia-related disorders, with notable links to overlapping neurodegenerative syndromes including “frontotemporal dementia” (30) and “multiple system atrophy” (18). Keywords like “mild cognitive impairment” (30) and “parkinsonism” (19) appeared frequently in proximity to both clinical and diagnostic research clusters (Figure 3).
Foundational terms in the overlay visualization of all keywords such as “dementia” (253 occurrences), “Alzheimer’s disease” (243), and “Parkinson’s disease” (194) appeared earliest in the timeline, represented by darker blue hues. In contrast, terms such as “visual hallucinations” (60), “neuropsychiatric symptoms” (79), and “prevalence” (59) appear in lighter yellow tones, indicating more recent research focus. Intermediate terms like “alpha-synuclein” (81), “mild cognitive impairment” (91), and “depression” (81) bridge both clinical and pathophysiological domains, reflecting the interdisciplinary progression of the field (Figure 4).
The most prevalent topic in the citation topic micro graph was “dementia,” encompassing 256 publications, followed closely by “Parkinson’s disease” with 224 articles. Beyond disease classification, a substantial number of articles (n = 47) were grouped under “dementia caregivers.” Two additional micro-topics, “Alzheimer’s mechanisms” and “ALS mechanisms,” were comparatively smaller, with 10 publications each (Figure 5).
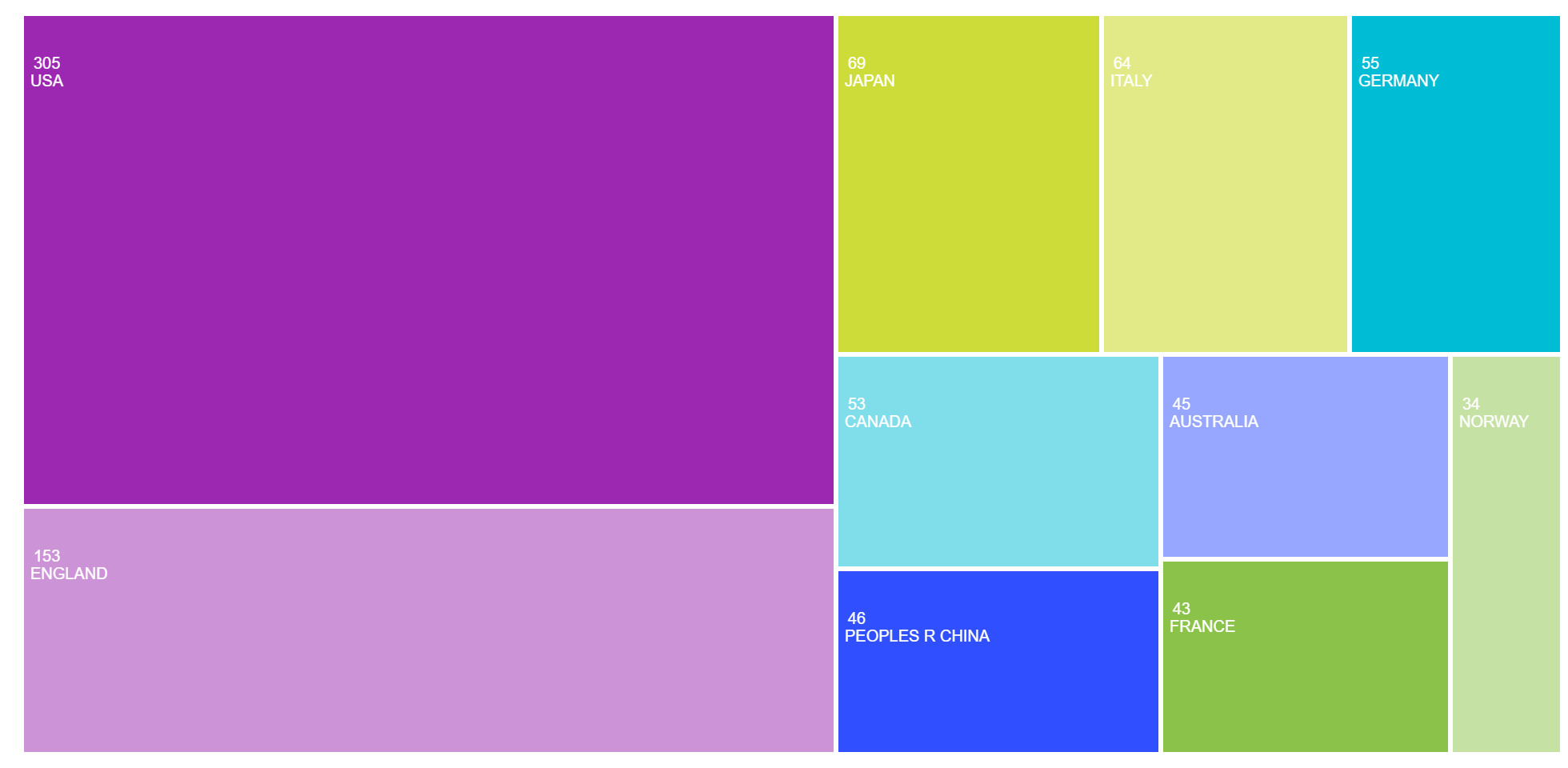
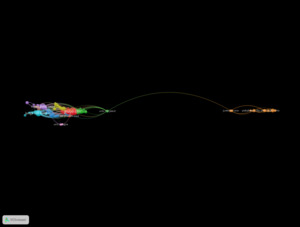
Surveying the major countries contributing to Lewy Body Dementia (LBD) research revealed a concentration of activity in high-income nations, with a notable transatlantic and East Asian presence (Figure 6). The United States led in publication volume with 305 articles, followed by England with 153, Japan with 69, and Italy and Germany with 64 and 55 publications, respectively. Canada (53), China (46, represented as “Peoples R China”), Australia (45), France (43), and Norway (34) also ranked among the top ten.
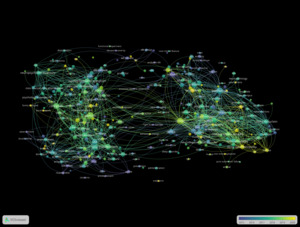
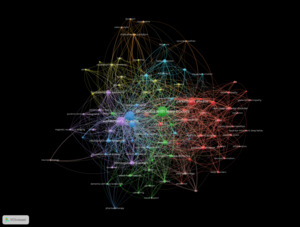
The co-authorship network at the institutional level revealed several major academic and clinical centres as key contributors to the field of Lewy Body Dementia (LBD) research (Figure 7). Mayo Clinic led in both productivity and impact, with 62 publications and 3,407 citations, and a total link strength of 36. King’s College London followed with 38 publications, 1,487 citations, and the highest total link strength (37).
Newcastle University also stood out, producing 42 publications with 3,231 citations and a link strength of 31. Other prominent institutions included University College London (25 publications; 1,221 citations; link strength 20), University of Pennsylvania (21; 1,880; 20), and the University of Cambridge (25; 2,170; 17). The Karolinska Institute, University of California, San Francisco, and University of Exeter each had more than 15 publications and demonstrated consistent link strength values around 16.
Smaller but well-integrated centers such as the Lewy Body Dementia Association (14 publications; link strength 14) and Banner Sun Health Research Institute (13 publications; link strength 12) also contributed meaningfully to collaborative knowledge production.
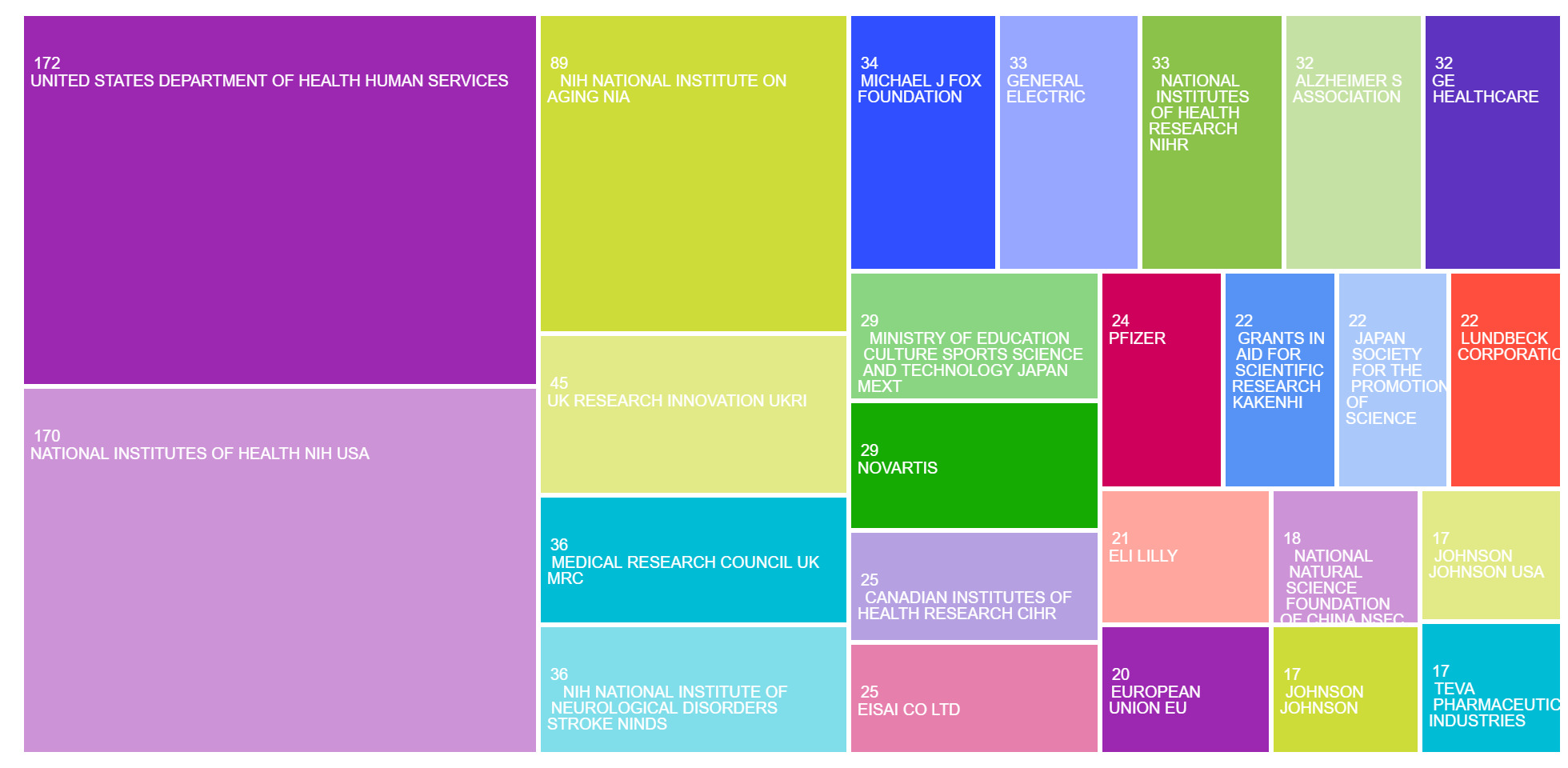
Analysis of funding sources revealed a strong concentration of financial support from U.S. government health agencies and major biomedical organizations (Figure 8). The United States Department of Health and Human Services was the most frequently cited funder, supporting 172 publications (22.3%), closely followed by the National Institutes of Health (NIH) with 170 publications (22.0%). Within NIH, the National Institute on Ageing (NIA) and the National Institute of Neurological Disorders and Stroke (NINDS) were prominent contributors, reflecting their focus on ageing and neurodegenerative disease mechanisms.
In the United Kingdom, UK Research and Innovation (UKRI) and the Medical Research Council (MRC) supported 45 and 36 publications, respectively, while the National Institute for Health Research (NIHR) accounted for another 33. Private and philanthropic organizations also played a notable role. The Michael J. Fox Foundation supported 34 studies, and the Alzheimer’s Association funded 32. Among private-sector contributors, General Electric (33), GE Healthcare (32), Novartis (29), Eisai Co., Ltd. (25), Pfizer (24), and Eli Lilly (21) were among the top funders, indicating strong industry engagement in LBD research.
Funding from Japanese sources—including the Ministry of Education, Culture, Sports, Science and Technology (MEXT), the Japan Society for the Promotion of Science, and the KAKENHI grants program—supported a combined total of over 70 studies. Additional support came from the European Union, the Canadian Institutes of Health Research (CIHR), and the National Natural Science Foundation of China (NSFC).
Discussion
Research on Lewy Body Dementia (LBD) has shown a consistently expanding area of study over the last forty years with publication activity rising significantly after 2016. This is likely because of the acknowledgement of LBD as a clinically unique condition that requires individual research. It’s also important to note that increased publicity and exposure of the wider global effects of ageing populations all over the world may have caused a steady rise in attention to the impact of neuropsychiatric symptoms associated with LBD on caregivers. Publication output reached its highest point in 2024, but the drop seen when comparing the projected value of publications produced in 2025 most likely indicates a bias in the time of year that such papers are published and indexed rather than a reduction in research efforts for LBD.
These thematic changes are reflected in the progression of language and emphasis. Initial studies focused on fundamental pathological concepts like “Parkinson’s disease,” “alpha-synuclein,” and “diagnosis.” Recent phrases like “care partner,” “quality of life,” and “support services” reflect the increasing focus on caregiving and psychosocial results in the field. The keyword and abstract overlay visualisations indicate that as the biological foundations of LBD were more comprehended, focus gradually shifted to clinical management, patient experiences, and supportive frameworks. Author keyword networks support this change, with frequently occurring terms like “depression,” “REM sleep behaviour disorder,” and “neuropsychiatric symptoms” creating central clusters, while more recent, contextually significant terms such as “caregiving” and “burden” have risen in importance.
Citation topic analysis further reinforces this development. Although “dementia” and “Parkinson’s disease” are still prevalent micro-level subjects, the rise of “dementia caregivers” as a separate citation cluster highlights an increasing academic dedication to exploring the human and relational aspects of LBD. This caregiver-centred focus now signifies an important subfield within the broader research environment, progressively enhancing investigation and partnership.
Research on LBD is primarily driven by wealthy countries, with the United States (305 publications) and the United Kingdom (153) at the forefront. Japan, Italy, and Germany also provide substantial contributions. Recent inputs from China and Australia suggest a growth in global engagement, likely driven by an ageing demographic and evolving national dementia strategies. Co-authorship networks demonstrate the clustering of countries with comparable health priorities: the U.S. and the U.K. frequently collaborate, while East Asian nations form unique but growing research partnerships.
At the institutional level, prominent centers like Mayo Clinic, King’s College London, and Newcastle University not only generate substantial research output but also support collaborative networks. In the meantime, dedicated organizations such as the Lewy Body Dementia Association are crucial for community involvement and advocacy-oriented research. This variety of institutions represents the field’s multidisciplinary character, encompassing clinical neuroscience, behavioral health, caregiving, and public health.
Funding trends validate this intricacy. U.S. federal agencies, mainly the NIH and NIA, offer most of the support, supplemented by contributions from the U.K., Canada, Japan, and the EU. Philanthropic entities like the Michael J. Fox Foundation and Alzheimer’s Association, together with private-sector supporters such as Novartis, Eisai, and Pfizer, demonstrate a shared interest in research related to diagnostics, therapeutics, and quality of life.
Conclusion
This bibliometric analysis highlights an increasing global research focus on the impact of neuropsychiatric symptoms of Lewy Body Dementia (LBD) on caregiver burden from 1986 to 2025, with the United States and England leading in publication volume and collaboration. The findings suggest a shift in the research emphasis with early studies focusing on pathology and diagnosis, while recent research has been on neuropsychiatric symptoms, caregiver burden, and long-term care. Similarly, keyword patterns and citation topics reflect a broader shift toward patient-centered and holistic approaches. Continued research, particularly on early and accurate diagnosis, is essential to improving outcomes for both individuals with LBD and their caregivers.

_in_the_analyzed_dataset_(n___770).png)
_in_lewy_body_dementia_research_articles.png)


_in_the_analyzed_dataset_(n___770).png)


_in_lewy_body_dementia_research_articles.png)