INTRODUCTION
BACKGROUND
Tuberculosis (TB) is an infectious disease caused by Mycobacterium Tuberculosis (MTB). Early recognition and diagnosis are fundamental in reducing transmission and death from the disease. Globally, about 10.6 million people fell ill with TB in 2021, an increase of 4.5% from 10.1 million in 2020.1 The TB incidence rate rose by 3.6% between 2020 and 2021, reversing declines of about 2% per year for most of the previous 2 decades.1 Nigeria accounted for one of the countries with the highest TB burden. The estimated epidemiological burden of TB in Nigeria was 219 per 100 000 populations with 64 per 100 000 population TB deaths.1 In a sample year (2019), unpublished data from the DOTS center in Lagos university teaching hospital (LUTH), where free GeneXpert testing and dispensation of anti-tuberculosis medications are carried out, showed that the number of patients with a presumptive diagnosis for TB who were screened using GeneXpert was 889, out of which 126 had a positive result and 763 had a negative result. That year the proportion of screened cases with a negative GeneXpert test was 86%, implying that the diagnosis of tuberculosis may have been missed in a large number of patients that were screened.
In the past, TB was frequently being diagnosed by direct sputum smear microscopy, because of the limited culture facilities and the long turn-around time involved with sputum culture which is typically the gold standard.2,3 The limitations of sputum smear microscopy include: a low sensitivity (50-60%), potential false positive results in individuals infected with non-tuberculous mycobacterium, potential false negative results in children, elderly and HIV infected patients.2 The World Health Organization (WHO) 2010 endorsed the GeneXpert test for use in endemic countries and declared it a major milestone for global TB diagnosis.1 A study done by Afsar et al found the sensitivity and specificity of GeneXpert to be 96.8 % and 99.3 %, respectively, compared to culture.3 The advantages that made GeneXpert more acceptable, are its higher sensitivity when compared to sputum smear microscopy and shorter period (2 hours) of obtaining the result when compared with culture.2 In 2011, WHO issued a policy statement recommending the use of the assay as a diagnostic tool for all people living with HIV who have signs and symptoms of TB, for people with unknown HIV status presenting with strong clinical evidence of HIV infection, for people who are seriously ill and suspected of having TB regardless of HIV status and those at risk of MDR TB2,4 However, its ability to detect mycobacterium is considerably affected by the quality of sputum produced by the patients.5 Sputum of good quality appearance (not salivary) with a volume of at least 2ml is required.5 This Xpert® MTB/RIF assay (GeneXpert) is a rapid molecular diagnostic and automated test that can detect both TB and rifampicin resistance, within two hours after starting the test.5 However, because of the poor method of sputum collection, the volume of sputum, the timing of sample collection, and delay in presentation of sample to the laboratory, sputum GeneXpert result may be negative.
Spontaneously produced sputum is the preferred specimen for confirming a diagnosis of pulmonary tuberculosis (PTB) as it is non-invasive and microbiological identification is higher from respiratory secretions. Suspected cases are expected to deeply expectorate from within the lungs so as to increase the possibility of having the mycobacterium in the submitted specimen.5 A properly produced sputum should include recently discharged material, with minimum amounts of oral or nasal material.5,6
Obtaining sputum of adequate quality may sometimes be a challenge. Such situations include adult patients with HIV who are sputum-scarce, and children.6 Inability to spontaneously produce sputum poses a diagnostic dilemma in patients with a presumptive diagnosis of tuberculosis which is defined as the presence of ≥ 2 clinical features like cough, fever, weight loss, etc. of > 2 weeks’ duration with any of these chest X-ray findings: consolidation, cavitation, nodular and miliary shadows in smear-negative cases.7 It is therefore imperative to seek other possible means of collecting sputum specimens in those patients with clinical and radiological suspicion of TB who are unable to expectorate sputum or have GeneXpert negative samples.7 On this backdrop, methods to increase the collection of sputum samples of adequate quality for the diagnosis of TB have been utilized each with its merits and demerits. These include sputum induction (SI), broncho-alveolar lavage (BAL), and gastric washings.7 On the one hand, specimen obtained via bronchoscopy is more representative than gastric washings and spontaneously expectorated sputum, however, it is an invasive procedure that requires more expertise, is not cost-effective, and is not readily available in developing countries.7
Sputum induction, on the other hand, has been used to obtain sputum samples for the diagnosis of different respiratory diseases, the example is seen in patients with cystic fibrosis and fungal respiratory infections. Regarding the diagnosis of pulmonary TB, it has been shown to be a cheap, safe, non-invasive procedure with greater patient comfort, suitable for all ages, and requires modest expertise.6,7 It has been shown to increase the diagnosis of TB by 34% with initiation of TB treatment.7 Compared to bronchoscopy, the yield of sputum induction is considered to be better than bronchoscopy in a study by McWilliams et al where results of three induced sputum tests were compared with bronchoscopy washings in 129 subjects with possible pulmonary tuberculosis.8
There has been a disproportionate increase in smear negative cases relative to smear positive TB cases in Nigeria.9 In a study done in Kano (Northwestern Nigeria) by Iliyasu et al, the proportion of sputum smear-negative HIV patients was 82.5%,10 while a previous study in Maiduguri (Northeastern Nigeria) by Moses et al reported a figure of 53% among TB/HIV co- infected patients.11 The implication of this is that there may be inadequate case detection of TB and hence can increase the burden (morbidity and mortality) of tuberculosis in Nigeria. The utility of sputum induction in improving the diagnosis of TB in the Nigerian context has not been extensively evaluated. This is even more pertinent in the Nigerian setting where there is also a high burden of HIV patients who also have TB. Also, there is no published data on the proportion of patients with TB who are unable to produce sputum in Nigeria. Furthermore, the limited availability of bronchoscopy services in Nigeria and other low and middle-income countries, makes induced sputum a more feasible approach with the potential to enhance TB diagnosis, treatment, and control.
Sputum induction is a procedure used for patients who have trouble producing sputum spontaneously so therefore it can aid the diagnosis of tuberculosis in patients who are unable to expectorate adequate sputum samples.12–15 Additionally, it is simple and relatively cost effective and if successful, often excludes the need for a bronchoscopy in patients who had an initial negative GeneXpert result.16,17
MATERIALS AND METHODS
STUDY LOCATION
The study was done at the Lagos University Teaching hospital (LUTH), a tertiary institution, with 761 beds, located in Lagos which is one of the largest economically important states in the country with an estimated population of 21 million as in 2015. Lagos is a cosmopolitan state and the commercial nerve center of the country. It is located in the South-Western part of Nigeria and a majority of the inhabitants are traders, artisans, private employees, and civil servants.
Participants recruited for the study are patients that presented to the respiratory clinic, emergency department and DOTS center, with respiratory symptoms, and some of whom were admitted into medical wards.
STUDY DESIGN
This study was a cross-sectional study.
SAMPLING TECHNIQUE
The sampling technique used was a non-probability sampling technique. A consecutive sampling of all patients that met the inclusion criteria was done.
STUDY DURATION
The study was carried out over a 12-month period from September 14th, 2021 to September 2022 following approval of the study protocol by the National Postgraduate Medical College.
STUDY POPULATION
The participants were inpatients and outpatients who consented and met the inclusion criteria with clinical features suggestive of PTB such as
-
cough of more than 2 weeks, with or without sputum
-
fever
-
hemoptysis
-
weight loss
-
night sweats
And had any of the following findings on chest imaging such as
-
consolidations predominantly in upper lung zones
-
cavitary lesions
-
centrilobular nodules
-
tree-in-bud appearance
-
miliary shadows
-
mediastinal lymph nodes
INCLUSION CRITERIA
Following brief history includes
-
Patient aged ≥18years with symptoms of TB
-
Patient not previously on treatment for TB
-
Patient that is hemodynamically stable
-
Patient who provided informed consent
-
Patient who was sputum scarce (produce < 2mls or unable to expectorate)
-
Patient with initial negative GeneXpert result on expectoration within preceding 4 weeks
EXCLUSION CRITERIA
Following history taking
-
Patients with uncontrolled asthma or chronic obstructive pulmonary disease and unstable heart failure.
-
Patients with active hemoptysis.
-
Patient that has had recent eye surgery (3-6weeks), recent abdominal surgery (< 6 weeks), unstable angina, or arrhythmias.
-
Patients already on tuberculosis treatment.
OUTCOME
Primary outcome
- The proportion of patients with positive GeneXpert test after sputum induction
Secondary outcome
-
Adverse effects relating to sampling procedure in sputum induction
-
The quality of sputum samples as measured by the Bartlett scores
SAMPLE SIZE DETERMINATION
The sample size was determined using Fisher’s statistical formula sample size.
Sample size N=Z2pq/d2
Z = standard deviation set at 1.96 which corresponds to a 95% confidence interval
P = percentage of the number of screened cases with a negative GeneXpert test for TB in LUTH in 2019
p = 0.86
q = 1-p
d = precision (margin of sampling error set at 10%) = 0.10
N= (1.96)2 x 0.14 x 0.86 ÷ (0.10)2= 46.25
Therefore, the sample size is approximately 47.
ETHICAL CONSIDERATIONS
Ethical approval for the study was obtained from the Health Research Ethics Committee of the Lagos University Teaching Hospital (LUTH-HREC), prior to the commencement of recruitment into the study (ADM/DCST/HREC/APP/4009).
INFORMED CONSENT
Written informed consent was obtained from all the study participants, prior to recruitment into the study. Information was provided in a written format, about the nature of the study, its objectives, potential benefits, risks, and the voluntary nature of participation. Participants were allowed to ask questions to ensure they understand the study protocol. Adequate time was given to allow participants to make an informed decision on participation.
VARIABLES
-
Clinical variables
a. Sociodemographic data (including age, sex/gender, etc.)
b. Smoking status (never-smoker, ever-smokers, ex-smoker)
c. Co-morbidity (HIV, hypertension, diabetes mellitus, others)
d. Symptoms of PTB (cough +sputum, fever, night sweats, weight loss, hemoptysis)
e. Examination findings: Anthropometry (e.g., weight, height, BMI, etc.)
-
Radiologic variables-
a. upper zones infiltrate/alveolar shadows/pneumonic changes
b. pulmonary nodules
c. cavitations
d. enlarged hilar nodes
e. pulmonary nodules
f. pneumonic lesion
g. military pattern
h. atelectasis
-
Laboratory Variables-Two step procedure
a. Bartlett scores (pus cells, epithelial cells, mucus)
b. Sputum adequacy (yes or no)
c. AFB (positive or negative
d. GeneXpert MTB (detected or not detected)
e. load (low, med, high)
f. RIF detected or not detected
Primary outcome variable would be positive GeneXpert test after sputum induction while secondary outcome would include side effects of sputum induction and adequacy of sputum following induction.
DATA COLLECTION
Participants were interviewed, using a designed proforma (Appendix 2). The following data were recorded; socio-demographic status (sex, age, marital status, occupation, level of education, height, weight, BMI) history of cough > 2 weeks, fever, hemoptysis, night sweats, sputum production, weight loss, dyspnea, HIV status, hypertension, diabetes, sickle cell, and smoking status. Smoking status was categorized into never-smokers who had never smoked as many as one cigarette per day for the duration of one year, ever-smokers who had smoked at least one cigarette per day for at least one year, ex-smokers who had stopped smoking for at least one year and current smokers who had smoked within the past year.4,5 Initial negative GeneXpert result; if available was recorded.
X-rays and computed tomography were reviewed under supervision by a radiologist and pathologies identified, which includes nodular, alveolar, or interstitial infiltrate predominantly affecting the upper zones, the presence of cavitation affecting the upper zones, enlarged hilar nodes, pneumonic lesion, atelectasis, and military pattern using a reporting sheet for standard findings in TB. The weight and height of each participant were measured and Body Mass Index (BMI) calculated. BMI was categorized according to WHO criteria as underweight(15-19.9kg/m2), normal weight (20 -24.9kg/m2), overweight (25- 29.9kg/m2), and obese >30 kg/m.2,4,6
Each participant was asked to expectorate a sample of sputum in the early morning from 8 am following instructions on breathing and encouragement, after which the participant underwent sputum induction.
EXPECTORATED SPUTUM
The participants were educated on the difference between sputum, saliva, or nasopharyngeal secretions and encouraged to produce a deep, productive cough from deep breaths into a sterile cup and labeled. After about 10mins, sputum induction was carried out for 20mins, and the samples produced were collected in a second sterile cup and labeled. The 2 specimens were taken to the lab in a small cooler, arriving within 1 hour of sample collection. The volume of sputum, appearance, and procedural side effects observed were documented in the proforma.
SPUTUM INDUCTION PROCEDURE
The protocol used was according to the European Respiratory Society Task Force for standardization of sputum induction.4,7
The procedure was done in an open area outside. A description of the procedure and instruction was given to each patient highlighting potential adverse effects. Adequate personal protective equipment was worn (N95 masks, face shield, lab coats with disposable gloves, and a distance of at least 2 meters from the participants was kept following starting).
First, the participants were asked to rinse their mouths with repeated gargling using bottled water to avoid contamination with debris. Then a pre-bronchodilator test using a peak flow meter with a disposable mouthpiece was done to assess the baseline. A safety limit of 350-700 L/m was used to proceed with the procedure. The best of three blows was selected using the standard procedure for measuring peak flow. Oxygen saturation was assessed and if >94%, the procedure was done. Next, 4 puffs of salbutamol (400µg), was inhaled by all participants to minimize bronchoconstrictive response to saline inhalation. After 10 minutes, a post-bronchodilator peak flow rate (PEF) was measured. The ultrasonic nebulizer was set to give an output of 1ml per min. The participants were instructed to inhale and exhale the mist of the nebulized 3% hypertonic saline solution through the disposable adult facemask. The inhalation of hypertonic saline was interrupted every 5 minutes, and the participants were asked to cough and expectorate sputum into a clean sterile sputum container. A total of 20min was allowed for the induction. The peak flow meter was assessed at 5 mins, 10 mins, 15 mins, and 20 mins intervals. If the PEF falls more than 20% from the post-salbutamol value or if symptoms develop, the procedure was stopped. Any side effect due to the procedure was recorded on the proforma using a check list of the possible side effects following nebulization. The total induction time, whether the process was completed or not and tolerated or not was documented. Volume of induced sputum was documented. Induction was considered successful if at least 2 ml of sputum is induced. The participants were closely monitored at all times during the procedure and a peak flow was assessed 1 hour after the end of sputum induction. Oxygen saturation was also assessed. Following the occurrence of potential adverse effects like wheezing, and persistent cough, hemoptysis was recorded at the end of the induction. There was no drop-in post-procedure peak flow rate by 20% or more from the post-salbutamol value after the induction. The procedure was never stopped and there was no need for rescue nebule salbutamol to relieve symptoms. There was no need for oxygen administration via an intranasal cannula on account of desaturation < 94%. Peak flow meter was monitored every 5 minutes for 1 hour. There was no need for admission of any of the participants into the accident and emergency for observation and treatment.
SAMPLE HANDLING
Permission to carry out the above procedures was obtained from the Head of the department of microbiology and the Head of the DOTS centre, at Lagos university teaching hospital (LUTH). The GeneXpert tests for the induced sputum were done for free in LUTH while GeneXpert tests for expectorated sputum were done at Randle General hospital in Lagos state, also for free. The cost of staining reagents and other study materials was covered by me.
Induced sputum samples and expectorated sputum samples where available, were collected in 2 sterile containers each. Each sputum sample collected was divided into 2 aliquots; one for GeneXpert testing, and the other for Ziehl-Neelsen and Gram’s staining for AFB, microscopy, and Bartlett`s scoring. The samples were transported within 1 hour of collection to the laboratory in the DOTS center in LUTH for processing while my research assistant took the expectorated sample for GeneXpert to Randle General Hospital. The processing of the samples was done by me with supervision by the clinical microbiologist. Volumes in mls (<2ml, 2-5ml, >5ml), as well as the physical appearance of both samples (salivary, mucoid, bloodstained, purulent), was noted, documented in the proforma
SAMPLE PREPARATION FOR GENEXPERT
The type of machine used in LUTH is the CEPHEID GeneXpert machine which uses a GeneXpert Dx system window.
Two parts of sample reagent were added to 1 part of sputum and then shaken vigorously for 10-20 mins. Mixture was then incubated for 10 minutes at room temperature, shaken vigorously for another 10-20 mins, and later incubated for another 5 mins until the sample was perfectly fluid. Using a plastic pipette, 2mls of the sample were transferred to the Xpert MTB/RIF cartridge, the lid closed, and the test was started within 30mins. The green light on the machine stops blinking after the cartridge is loaded. The lights were turned off at the end of the test, the system released the door lock, and the cartridge was removed and discarded in a biohazard discard bag. The process took about 2 hours and result of the test was reported on the GeneXpert software as “MTB detected or MTB not detected”. Rifampicin resistance was reported as “Rif resistance detected or Rif resistance not detected”.
GRAM SMEAR PREPARATION FOR BARTLETT SCORING
Using a platinum wire loop, sputum was placed over the central area of the slide. A wooden applicator was used to spread the sample to an even-thin film over a circle of about 20mm in diameter. The slide was allowed to air dry for 30 mins, and then heat-fixed over a gentle flame. Crystal violet stain was added over the fixed slide, and allowed to stand for at least 60 seconds after which the stain was poured off and the excess was gently rinsed with a stream of water. Next, the smear was stained with Lugol`s iodine, enough to cover the fixed slide, and allowed to stand for at least 60 seconds. Acetone (decolorizer) was then trickled down the slide and rinsed off with water immediately. Counterstaining with Safranin solution was done for 40 to 60 seconds. The solution was rinsed off with water, then the back of the slide was cleaned, and the slide was allowed to air dry and then examined using a 40X objective.
ADEQUACY OF INDUCED SPUTUM
The gram-stained slides were viewed under a light microscope with a low power magnification (magnification of objective lens at x5). The number of neutrophils and epithelial cells was counted. Adequacy of sputum was determined using a standard checklist, according to the criteria of Bartlett, and documented in the proforma.
SMEAR PREPARATION ZIEHL-NEELSEN STAINING
Using a platinum wire loop sputum was placed over the central area of the slide. A wooden applicator was used to spread the sample to an even-thin film over a circle of about 20mm in diameter. The slide was then allowed to air dry then heat- fixed over a gentle flame. The smear was covered with carbol fuchsin stain and heat was gently applied until the vapor just begins to rise. The heated stain was allowed to remain on the slide for 5 minutes, then rinsed off with clean water. Next, the smear was covered with 3% v/v acid alcohol (or 20% sulphuric acid) for 2-5 minutes until the smear was sufficiently decolorized, i.e. pale pink, and rinsed afterward. The slide was then covered with methylene blue for 1-2 minutes and rinsed off with clean water. back of the slide was cleaned and the slide was allowed to air dry, then examined using a 100X oil immersion objective).
DIAGNOSIS OF PTB
Any red bacilli seen in the Ziehl-Neelson stained smear was reported as 'AFB positive`.18,19
Diagnosis of PTB was made when MTB was detected by GeneXpert and rifampicin resistance and MTB load were noted.
INFECTION PREVENTION AND CONTROL
Standard precaution was used for handling of all biological specimens. Wearing of protective disposable gloves, laboratory coats, and an N95 respirator when handling specimens and reagents was compulsory. Hands were washed thoroughly after handling specimens and test reagents. All biological wastes were put in a biohazard bag and autoclaved.
DATA HANDLING
Data obtained was entered into Microsoft excel and then analyzed using Statistical Package for Social Sciences (SPSS IBM) version 25. Categorical variables (level of education, occupation, marital status, patient that are sputum scarce or have negative GeneXpert, symptoms of TB, radiological findings, Zeihl Neelson stain, and GeneXpert test) were presented using frequency and percentages. Numeric variables (age, height, weight, smoking status) were represented using mean and standard deviation. A test of normality would be done using the Kolmogorov-Smirnov test. Association between categorical variables will be performed using chi-square or Fisher’s exact test. The comparison between the adequacy of sputum was carried out using the McNemer test. Mean comparison between the two groups will be carried out using the independent student T-test while median comparison will be assessed the using Mann-Whitney U-test. A p-value of less than 0.05 will be considered significant at 95% confidence interval for all associations.
RESULTS
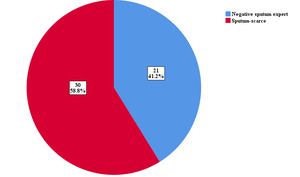
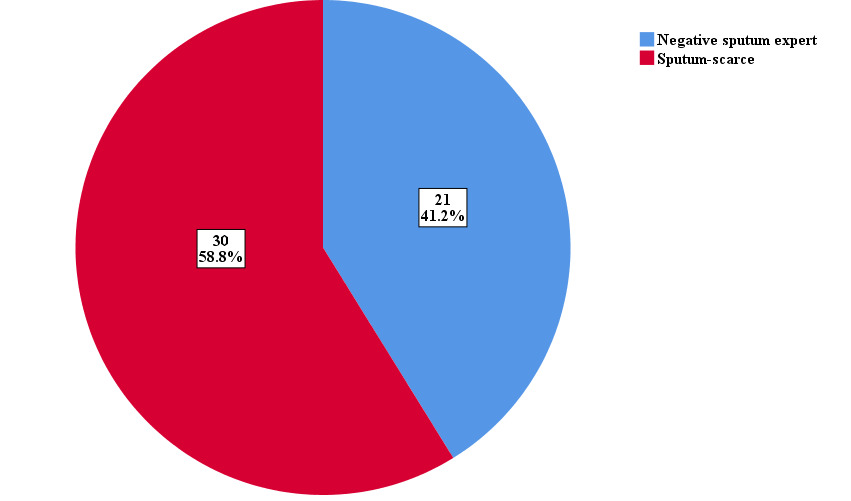
A total of 60 patients who consented and met the inclusion criteria were invited to participate in the study following the proposal approval on 14th September 2021 till September 2022. Sputum induction failed in 8 patients and they were excluded from the analysis, and one patient had a low pre-procedure Peak flow rate (220 mLs) and was also excluded from the study. The subgroup of patients involved in the study is as shown in Figure 1.
A total of 102 samples; 51 induced sputum samples and 51 expectorated samples following instructions and encouragement, were evaluated. The characteristics of the study population are shown in Table 1. The mean age of all patients was 46.69± 15.3 years, 60.8% were males, and 39.2% were females. There was no statistically significant difference in the socio-demographic characteristics between the 2 groups.
Table 2 shows the clinical characteristics. The most common symptoms were cough 49 (96.1%), weight loss 37 (72.5%), and fever 29 (56.9%). The majority of the patients had no comorbidities. Five patients (9.8%) were HIV positive or had hypertension, and 2 (3.9%) had diabetes and sickle cell disease, respectively.
The radiologic patterns of the patients are as shown in Table 3.
A total of 45 of 102 sputum samples collected was adequate according to bartlett’s criteria. Sixteen (31.4%) from the expectorated samples and 29 (56.9%) from the induced sputum samples. Induced sputum had more adequate samples with a significant p value 0.001, which is shown below in Table 4.
Table 5 shows to the comparison of sputum adequacy between induced sputum and expectorated sputum, and the relationship of adequate sputum sample and positive TB GeneXpert.
Mycobacterium detection was higher using GeneXpert than with AFB, and induced sputum gave a higher yield than expectorated sputum (Table 6). This increased yield in tuberculosis diagnosis following sputum induction in both subgroups of patients (negative GeneXpert and sputum-scarce) is seen in Figure 2.
Table 7 shows the agreement between positive GeneXpert test on induced compared to expectorated sputum. There was a statistically significant difference, p=0.004.
Side effects following sputum induction were seen in 15 of the 51 patients (29.4 %) that were induced. The commonest side effects seen in sputum induction were cough, breathlessness, and wheezing, 8(53.3%), 3(20%), and 2(13.3%) respectively. All side effects were reported after the induction, non were noticed during the procedure. They all resolved in less than 20 minutes within 1 hour of observation and there was no need for emergency review.
All patients with a positive GeneXpert after sputum induction were started on tuberculosis treatment immediately. The basis of starting treatment included a positive GeneXpert and clinical and radiological findings in keeping with TB.
Fourteen (27.5%) induced sputum samples gave a positive GeneXpert while 37 (72.5%) gave a negative GeneXpert. Six (16.2%) patients with a negative GeneXpert on induction were clinically diagnosed as PTB; based on clinical and radiological findings combined with a positive clinical response to empiric anti-tuberculosis therapy.
Three (5.9%) expectorated sputum samples gave a positive GeneXpert while 48(94.1%) were negative. Thirty-one (56.3%) had an alternate diagnosis of non-active TB etiology as shown in Table 8. The sensitivity, of sputum induction in producing samples for TB diagnosis is 70.0%, while that of sputum expectoration following instruction is 15.1%.
DISCUSSION
The findings of this study suggest that there may be improved yield in TB diagnosis from induced sputum compared to expectorated sputum. The difference between the diagnostic yield of TB using induced sputum compared with expectorated sputum was evaluated by conducting a cross-sectional study among patients who are sputum scarce or had an initial negative GeneXpert that presented to Lagos University Teaching Hospital during the period of study.
We aimed to determine the diagnostic yield of induced sputum compared to expectorated sputum, the efficacy of sputum induction to obtain adequate lower respiratory tract sputum samples, and the frequency of side effects following sputum induction.
Induced sputum produced more adequate samples for testing TB using Bartlett’s criteria than expectorated sputum. In addition, in our study, more sputum samples produced following induction had epithelial cell counts that were < 10/low power field magnification (LPF) compared to expectorated sample, however, the range of neutrophil cells/LPF seen in both samples were approximately equal and a lesser number of induced and expectorated sputum samples had a neutrophil count that was >25/LPF. The difference between induced sputum and expectorated sputum epithelial cells and mucous cells was statistically significant, implying deeper expectoration following induction and hence lesser oropharyngeal contamination.18,19 This finding is similar to other studies that evaluated usefulness of sputum induction with hypertonic saline in real clinical practice for bacterial yield in active TB.19-26 Using their finding, they defined adequate sputum following induction as sputum sample that had < 25 squamous epithelial cells/LPF and a high neutrophil count > 25cells /LPF.26 Furthermore, this finding was also similar to those done in previous studies where there was a positive association between sputum leukocytosis and mycobacterial positivity.10–15 The similarity in these studies with the present study was the performance of microscopic examination of the cellular components in a Grams 'stained smear of the specimen, seen under LPF.
Then again, it was shown that there was a significant difference between the diagnostic yield of tuberculosis using induced sputum compared to expectorated sputum. The overall yield of GeneXpert positive-TB in both subgroups of patients, after sputum induction, is 21.6%. This increase in yield in TB diagnosis agrees with the findings of a study done in India which showed a 34 % increase in yield for TB diagnosis.2 The similarity in this study may be that induced sputum samples were gotten from patients with clinical and radiological features in keeping with TB and the same duration of nebulization was employed. The study found that sputum induction was successful in 95% of patients who could not expectorate adequate sputum, and this was similar to the present findings. Another study done by Khan et al showed a similar increase in the diagnosis of TB following sputum induction.16 Unlike the present study, the study was a retrospective study carried out on patients with scarce sputum, and culture was done on most induced sputum samples and few sputum specimens had a GeneXpert test performed. Here the sensitivity of sputum induction in diagnosing TB was 52%.16 This present study shows a 71% sensitivity of sputum induction in diagnosing TB. A high percentage here could be a result of more sputum samples being analyzed with GeneXpert.
Just as important, all the patients tolerated the procedure well with 15(29.4%) patients developing side effects. Coughing for a few mins post-procedure was the commonest side effect. In other studies, the majority of the patients also tolerated the sputum induction procedure well. Other studies showed that (15.90%) had repeated coughs with 5 cases developing bronchospasm requiring salbutamol nebulization.17–21 This could have resulted from the pre-treatment with a bronchodilator not being done prior to the procedure unlike in the present study.22 Other side effects noted in our study include breathlessness, wheezing, and nausea which all resolved spontaneously without any specific treatment. Same findings could be seen in other studies that complained of nausea and vomiting as the commonest side effect, which settled without any specific therapy.2,5 Here, the same milder concentration of hypertonic saline was used in the procedure.
The study showed no statistically significant difference in the sociodemographic characteristics of the patients, but a majority of patients recruited had a higher level of occupation and high educational skills. This finding would raise the index of suspicion of TB in people who are not of low economic status. Additionally, BMI was mostly normal among the study patients.
CONCLUSIONS
This study revealed a significantly higher yield of tuberculosis diagnosis following sputum induction with minimal side effects. It suggested a significant difference in the diagnostic yield of tuberculosis using induced sputum when compared to expectorated sputum from a suspected patient who has scarce sputum or initial negative GeneXpert.
RECOMMENDATION
Sputum induction is a safe and good complementary strategy to obtain sputum samples and thus should be done in our various DOTS centre following an initial negative GeneXpert result in suspected patients, also in sputum-scarce patients.
STUDY LIMITATIONS
Each pair of GeneXpert tests (expectorated sputum and induced sputum) were ran at different Government facilities that offer free GeneXpert testing as one test is allowed for each person at a particular facility for a specific time duration.
STRENGTH OF STUDY
This is a cross-sectional study that was done on inpatients admitted with symptoms and imaging findings suggesting TB.