To the Editor: Over the past decade, the management of atopic dermatitis (AD) has evolved with guidelines emphasizing a multifaceted and individualized approach. The American Academy of Dermatology (AAD) most recently updated their clinical guidelines addressing the safety and efficacy of AD treatments in 2023.1 These recommendations advocate for a stepwise framework that considers disease severity, patient preferences, and comorbidities. Management begins with topical therapy for acute flares and escalates to systemic therapies as needed.2
Topical corticosteroids (TCS) remain the cornerstone of AD management, followed by steroid-sparing topical agents, such as topical calcineurin inhibitors (TCI) and phosphodiesterase-4 (PDE4) inhibitors. The AAD cautions against the general and long-term use of TCS and oral immunosuppressants and only recommends their use as a last resort when other treatments have failed.1 This study analyzes the trends in prescribed treatment regimens for atopic dermatitis from 2013 to 2019.
The National Ambulatory Medical Care Survey (NAMCS), a cross-sectional survey of visits to nonfederal, ambulatory-based US physicians, was utilized to identify subjects who sought ambulatory treatment for AD from 2013 to 2019. AD patients were identified using the International Classification of Diseases, Ninth Edition (ICD-9) code 6918 and the International Classification of Diseases, Tenth Edition (ICD-10) code L209. Patients under the age of 15 and those without prescription management were excluded from the study. A total of 162 AD patients with active prescriptions were analyzed using STATA software.
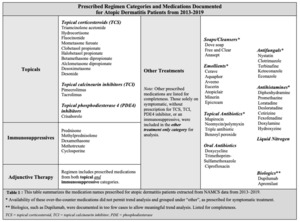
A Chi-square test was conducted to assess the relationship between prescribed medication regimens for atopic dermatitis and the year of prescription. Four categories of medications were analyzed: topical therapy only, immunosuppressant (IMS) therapy only, adjunctive therapy, and other treatment only (Table 1). Prescriptions were further stratified by single-agent and multi-agent regimens and the medication type (Table 2,3).
The Chi-square test revealed a significant association between prescribed regimens and the prescribed year (χ² (15) = 38.85, p = 0.001), indicating that the distribution of prescribed regimens has changed over this time. The steady use of topical therapy, combined with a reduction in immunosuppressive therapy from 2016 onwards, reflects a shift in clinical practice towards standardized guidelines of care to reduce long-term adverse effects.
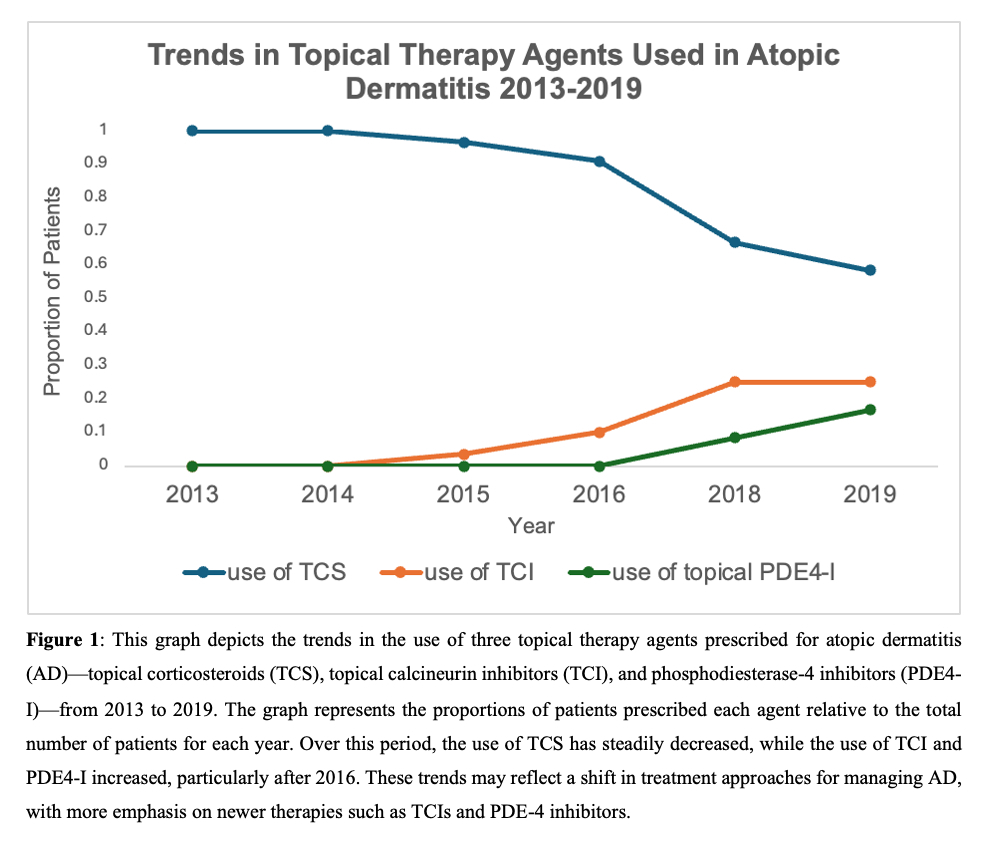
The preferred choice of topical agents prescribed in treatment regimens was also assessed. Figure 2 shows the proportion of patients prescribed each agent relative to the total number of patients per year. During this period, the use of TCS steadily decreased, while the use of TCI and PDE4 inhibitors increased, particularly after 2016. These trends reflect a growing preference for prescribing topical steroid-sparing agents over TCS.
In summary, this study demonstrates a significant shift in prescribing patterns for AD from 2013 to 2019. These findings suggest that treatment strategies for AD continue to evolve in response to emerging therapies and an improved understanding of the long-term effects of both topical and systemic corticosteroids, in alignment with the AAD guidelines.
Acknowledgements
Completion of our research review would not been possible without the support and guidance of Dr. Wu.
Funding sources
None
Conflicts of Interest
Dr. Jashin Wu is or has been an investigator, consultant, or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Bausch Health, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Codex Labs, Dermavant, DermTech, Dr. Reddy’s Laboratories, Eli Lilly, EPI Health, Galderma, Incyte, Janssen, LEO Pharma, Mindera, Novartis, Pfizer, Regeneron, Samsung Bioepis, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, and Zerigo Health. All other co-authors have no conflicts of interest to disclose.
IRB approval status
Not applicable.
Patient consent
Not applicable.
Reprint requests
Jashin Wu, MD