Introduction
Abdominal aortic aneurysms (AAA), the abnormal localized dilation of the lower aorta, represents a relatively common diagnosis with 2.5 to 6.5 diagnoses per 1,000 individuals in the US.1 Although often complicated by atypical presentations, the identification of AAA represents a task of the utmost importance, due to the condition’s potential complications, including rupturing, and lethal potential Although common symptoms of AAA include abdominal pain, back pain, and hypotension, many patients do not present with these indications. A high index of suspicion, patient history collection, and thorough physical examinations will help the physician make the correct diagnosis. For example, a full abdominal exam, assessing for rigidity, can be crucial in forming a diagnosis for AAA. There are several risk factors regarding the development of AAA, including smoking, age older than 65, male gender, and family history.2 The introduction of population screening, via ultrasounds, of all men who have ever smoked, are between 65-75 years old, and have been diagnosed or at risk of developing endovascular repair (EVAR) have resulted in the continued decrease in the incidence of ruptured AAA’s; this is largely due to an increase in intact-AAA repairs.3 These advances have spearheaded improvements in long-term survival rates regarding AAA. In the majority of cases, a AAA will occur between the renal arteries and the inferior mesenteric arteries.4 Regarding initial AAA diagnosis, and the condition’s atypical presentation, several cases have been documented where AAA presents as septic shock, leading to an inaccurate diagnosis.5 It is therefore important to always include AAA rupture in the differential possibilities of diagnosis, especially in cases where patients present with hypotension and an altered mental status. The Rapid Ultrasound for Shock and Hypotension (RUSH) exam has been shown to be useful in identifying causes of hypotension in altered patients. This contrasts with the Focused Assessment with Sonography for Trauma (FAST) exam, which alone may not identify retroperitoneal bleeding and does not visualize the aorta.6 In this report the authors present the case of a 72-year-old male with a ruptured infrarenal AAA, complicated by atypical presentation.
Case Presentation
A 72-year-old male with a past medical history of tobacco use and asthma presents to a local freestanding emergency department (ED) with a chief complaint of dyspnea and left sided chest wall pain after falling in the bathroom due to dizziness. Patient denied head injury, loss of consciousness, chest pain, palpitations, cough, fever, chills, nausea, vomiting, leg swelling, weakness, back pain, abdominal pain, recent hospitalizations, recent travel, or sick contacts. Following a physical exam, the patient was noted to be “toxic appearing,” in moderate respiratory distress, dyspneic, and tachypneic, but with clear lung sounds bilaterally. Abdominal exam areas were soft, non-tender, and non-distended. Initial vital signs read as follows: heart rate (HR) of 134bpm, respiratory rate (RR) of 21 bpm, blood pressure (BP) of 84/59 mmHg, oxygen saturation (SaO2) of 89% on room air, and temperature of 36.20C. In response to the presenting complaints, findings in the physical examination, and vital signs, the patient was immediately sepsis alerted and put on 4 liters per minute (LPM) oxygen via nasal cannula and received 30 ml/kg of normal saline and ceftriaxone, while a lab workup was requested. Laboratory evaluation revealed several notable findings: lactic acid 9.0 mmol/L, CO2 20 mmol/L, anion gap 21.4 mmol/L, creatinine 1.88 mg/dL, eGFR 35, glucose 268 mg/dL, troponin 0.067 ng/mL, d-dimer 9.4 mg/L, WBC 28.8 × 109/L, and hemoglobin 13.7 g/dL. Electrocardiogram (EKG) showed sinus tachycardia without ST elevation myocardial infarction (STEMI). Additionally, the patient’s FAST exam was negative. The patient’s SaO2 and blood pressure improved following the administration of oxygen and fluids. After initial treatment and stabilization, the patient was subsequently transferred to the main ED at a community hospital for further evaluation.
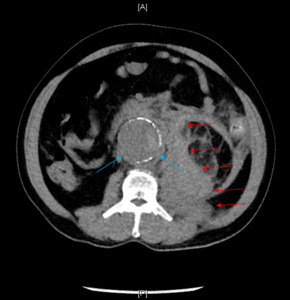
Upon arrival at the main ED, the patient was noted to be alert and oriented, ill appearing, tachycardic, and tachypneic. Upon further questioning, the patient stated he started having left lower quadrant abdominal pain and left flank pain that evening. The patient reiterated that the injury was sustained via a fall in a bathroom following sudden dizziness. He denied sustaining a head injury or syncope. Repeat vital signs showed HR 135bpm, RR 39bpm, BP 102/76 mmHg, SaO2 100% while on 4 LPM oxygen via a nasal cannula, and a temperature of 36.10C. In the repeat physical exam notable abdominal distention without tenderness was noted. Computed Tomography (CT) of the abdomen and pelvis was ordered for further evaluation. Given the patient’s low glomerular filtration rate, intravenous contrast was not utilized due to institutional protocol. Therefore, a non-contrast abdominal/pelvic CT was ordered to evaluate for abdominal distention and a ventilation-perfusion scan was ordered to evaluate for pulmonary embolism (PE). Repeat lactic acid was measured to be 6.0 mmol/L. Abdominal/pelvic CT showed a ruptured 5.5 cm infrarenal abdominal aortic aneurysm with peri-aortic and left retroperitoneal hemorrhage (Figure 1). Vascular surgery was immediately consulted, and the patient was taken to the operating room for emergent endovascular aneurysm repair (EVAR). The Intensive Care Unit (ICU) was also consulted for patient admission and further management. Post-operatively, the patient developed hyperglycemia, lactic acidosis, non-STEMI due to demand ischemia, acute respiratory distress syndrome (ARDS), intra-abdominal hypertension, acute blood loss anemia, acute kidney injury, and systemic inflammatory response syndrome (SIRS). The patient was aggressively treated, and was eventually able to be weaned off the ventilator, extubated and downgraded from the ICU after six days. After six more days of medical management, the patient was discharged in stable condition on hospital day 12 to a skilled nursing facility with a vascular surgery follow-up.
Discussion
The presented case underscores the importance of taking exhaustive health history and performing thorough and appropriate physical exams. The presenting patient had key risk factors for the occurrence of AAA: a history of smoking and old age.2 It should be highlighted that the history taken at the freestanding ED was different than the one taken at the main ED. Abdominal distention was not noted on the initial physical exam; an indication particularly difficult to notice because the patient did not have any abdominal tenderness during the initial abdominal exam. Although, the FAST exam was negative, a rather unsurprising discovery considering that a ruptured AAA would most likely cause a retroperitoneal bleed, perhaps the RUSH exam would have been more useful in the context of altered mental status and hypotension with no clear cause. The only limitation of performing the RUSH exam would usually be a patient’s body habitus.6 It is essential to always keep a wide differential diagnosis; this patient initially appeared to be septic with severe lactic acidosis, however upon further examination indications for AAA were observed and the correct, and a potentially life-saving diagnosis, was made. Therefore, looking for sources of infection was appropriate; however, searching for alternative causes to explain symptoms is equally important to ensure an all-encompassing diagnosis.
Conclusion
Prompt identification of an AAA rupture is paramount in cases of altered mental status and hypotension. In cases of hypotension without a clear cause, performing thorough history taking and analysis, detailed physical examinations, and knowledge regarding the utilization of the RUSH exam are paramount in detecting AAA ruptures. In the case presented, the distended abdomen led to the ordering of the abdominal/pelvic CT, which revealed additional indications, leading to the ultimate, and correct, diagnosis.